Beta Agonist Lung Injury TrIal-2 (BALTI-2) trial protocol: a randomised, double-blind, placebo-controlled of intravenous infusion of salbutamol in the acute respiratory distress syndrome
- PMID: 21554679
- PMCID: PMC3113985
- DOI: 10.1186/1745-6215-12-113
Beta Agonist Lung Injury TrIal-2 (BALTI-2) trial protocol: a randomised, double-blind, placebo-controlled of intravenous infusion of salbutamol in the acute respiratory distress syndrome
Abstract
Background: The acute respiratory distress syndrome (ARDS) is a common cause of respiratory failure in critically ill patients. Experimental studies suggest that treatment with beta agonists may be helpful in ARDS. The Beta Agonist Lung Injury TrIal (BALTI-2) is a multicentre, pragmatic, randomised, double-blind, placebo-controlled clinical trial which aims to determine if sustained treatment with intravenous (IV) salbutamol will improve survival in ARDS.
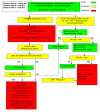
Methods/design: Patients fulfilling the American-European Consensus Conference Definition of ARDS will be randomised in a 1:1 ratio to receive an IV infusion either of salbutamol (15 μg kg ideal body weight-1 hr-1) or placebo (0.9% sodium chloride solution), for a maximum of seven days. Allocation to randomised groups will use minimisation to ensure balance with respect to hospital of recruitment, age group (<64, 65-84, >85 years) and PaO2/FiO2 ratio (≤6.7, 6.8- 13.2, ≥13.3 kPa). Data will be recorded by participating ICUs until hospital discharge, and all surviving patients will be followed up by post at six and twelve months post randomisation. The primary outcome is mortality at 28 days after randomisation; secondary outcomes are mortality in ICU, mortality in hospital, number of ventilator-free days, number of organ failure-free days, mortality at twelve months post-randomisation, quality of life at six and twelve months, length of stay in ICU, length of stay in hospital, adverse effects (tachycardia, arrhythmia or other side effects sufficient to stop treatment drug). 1,334 patients will be recruited from about fifty ICUs in the UK. An economic evaluation will be conducted alongside the trial.
Trial registration: Current Controlled Trials ISRCTN38366450.
Figures
References
-
- Bernard GR, Artigas A, Brigham KL, Carlet J, Falke K, Hudson L, Lamy M, Legall JR, Morris A, Spragg R. The American-European Consensus Conference on ARDS. Definitions, mechanisms, relevant outcomes, and clinical trial coordination. Am J Respir Crit Care Med. 1994;149(3 Pt 1):818–824. - PubMed
-
- Brun-Buisson C, Minelli C, Bertolini G, Brazzi L, Pimentel J, Lewandowski K, Bion J, Romand JA, Villar J, Thorsteinsson A. et al.Epidemiology and outcome of acute lung injury in European intensive care units. Results from the ALIVE study. Intensive Care Med. 2004;30(1):51–61. doi: 10.1007/s00134-003-2022-6. - DOI - PubMed
-
- Intensive Care National Audit Research Centre. Incidence and outcome of acute respiratory failure - ICNARC data on file. 2005.
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources