Target for improvement: a cluster randomised trial of public involvement in quality-indicator prioritisation (intervention development and study protocol)
- PMID: 21554691
- PMCID: PMC3118228
- DOI: 10.1186/1748-5908-6-45
Target for improvement: a cluster randomised trial of public involvement in quality-indicator prioritisation (intervention development and study protocol)
Abstract
Background: Public priorities for improvement often differ from those of clinicians and managers. Public involvement has been proposed as a way to bridge the gap between professional and public clinical care priorities but has not been studied in the context of quality-indicator choice. Our objective is to assess the feasibility and impact of public involvement on quality-indicator choice and agreement with public priorities.
Methods: We will conduct a cluster randomised controlled trial comparing quality-indicator prioritisation with and without public involvement. In preparation for the trial, we developed a 'menu' of quality indicators, based on a systematic review of existing validated indicator sets. Participants (public representatives, clinicians, and managers) will be recruited from six participating sites. In intervention sites, public representatives will be involved through direct participation (public representatives, clinicians, and managers will deliberate together to agree on quality-indicator choice and use) and consultation (individual public recommendations for improvement will be collected and presented to decision makers). In control sites, only clinicians and managers will take part in the prioritisation process. Data on quality-indicator choice and intended use will be collected. Our primary outcome will compare quality-indicator choice and agreement with public priorities between intervention and control groups. A process evaluation based on direct observation, videorecording, and participants' assessment will be conducted to help explain the study's results. The marginal cost of public involvement will also be assessed.
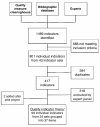
Discussion: We identified 801 quality indicators that met our inclusion criteria. An expert panel agreed on a final set of 37 items containing validated quality indicators relevant for chronic disease prevention and management in primary care. We pilot tested our public-involvement intervention with 27 participants (11 public representatives and 16 clinicians and managers) and our study instruments with an additional 21 participants, which demonstrated the feasibility of the intervention and generated important insights and adaptations to engage public representatives more effectively. To our knowledge, this study is the first trial of public involvement in quality-indicator prioritisation, and its results could foster more effective upstream engagement of patients and the public in clinical practice improvement.
Trial registration: NTR2496 (Netherlands National Trial Register, http://www.trialregister.nl).
Figures


References
-
- Braspenning J, Campbell S, Grol R. In: Improving patient care: the implementation of change in clinical practice. Grol R, Wensing M, Eccles M, editor. iv. New York City: Elsevier Butterworth Heinemann; 2005. Measuring changes in patient care: development and use of indicators; p. 290.
-
- Campbell SM, Roland MO, Quayle JA, Buetow SA, Shekelle PG. Quality indicators for general practice: which ones can general practitioners and health authority managers agree are important and how useful are they? J Public Health. 1998;20:414–421. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Miscellaneous

