Expression of mutant alpha-synuclein modulates microglial phenotype in vitro
- PMID: 21554732
- PMCID: PMC3104357
- DOI: 10.1186/1742-2094-8-44
Expression of mutant alpha-synuclein modulates microglial phenotype in vitro
Abstract
Background: Increased reactive microglia are a histological characteristic of Parkinson's disease (PD) brains, positively correlating with levels of deposited α-synuclein protein. This suggests that microglial-mediated inflammatory events may contribute to disease pathophysiology. Mutations in the gene coding for α-synuclein lead to a familial form of PD. Based upon our prior findings that α-synuclein expression regulates microglial phenotype we hypothesized that expression of mutant forms of the protein may contribute to the reactive microgliosis characteristic of PD brains.
Methods: To quantify the effects of wild type and mutant α-synuclein over-expression on microglial phenotype a murine microglial cell line, BV2, was transiently transfected to express human wild type (WT), and mutant α-synuclein (A30P and A53T) proteins. Transfected cells were used to assess changes in microglia phenotype via Western blot analysis, ELISA, phagocytosis, and neurotoxicity assays.
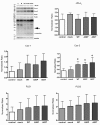
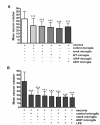
Results: As expected, over-expression of α-synuclein induced a reactive phenotype in the transfected cells. Expression of α-synuclein increased protein levels of cycloxygenase-2 (Cox-2). Transfected cells demonstrated increased secretion of the proinflammatory cytokines, tumor necrosis factor-α (TNF-α) and interleukin-6 (IL-6), as well as increased nitric oxide production. Transfected cells also had impaired phagocytic ability correlating with decreased protein levels of lysosomal-associated membrane protein 1 (LAMP-1). In spite of the increased cytokine secretion profile, the transfected cells did not exhibit increased neurotoxic ability above control non-transfected BV2 cells in neuron-microglia co-cultures.
Conclusions: These data demonstrated that over-expression of α-synuclein drives microglial cells into a form of reactive phenotype characterized by elevated levels of arachidonic acid metabolizing enzymes, cytokine secretion, and reactive nitrogen species secretion all superimposed upon impaired phagocytic potential.
Figures
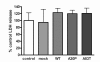


References
-
- Fischer HG, Reichmann G. Brain dendritic cells and macrophages/microglia in central nervous system inflammation. J Immunol. 2001;166:2717–26. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

