Selection of drug resistant mutants from random library of Plasmodium falciparum dihydrofolate reductase in Plasmodium berghei model
- PMID: 21554743
- PMCID: PMC3100258
- DOI: 10.1186/1475-2875-10-119
Selection of drug resistant mutants from random library of Plasmodium falciparum dihydrofolate reductase in Plasmodium berghei model
Abstract
Background: The prevalence of drug resistance amongst the human malaria Plasmodium species has most commonly been associated with genomic mutation within the parasites. This phenomenon necessitates evolutionary predictive studies of possible resistance mutations, which may occur when a new drug is introduced. Therefore, identification of possible new Plasmodium falciparum dihydrofolate reductase (PfDHFR) mutants that confer resistance to antifolate drugs is essential in the process of antifolate anti-malarial drug development.
Methods: A system to identify mutations in Pfdhfr gene that confer antifolate drug resistance using an animal Plasmodium parasite model was developed. By using error-prone PCR and Plasmodium transfection technologies, libraries of Pfdhfr mutant were generated and then episomally transfected to Plasmodium berghei parasites, from which pyrimethamine-resistant PfDHFR mutants were selected.
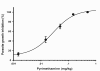
Results: The principal mutation found from this experiment was S108N, coincident with the first pyrimethamine-resistance mutation isolated from the field. A transgenic P. berghei, in which endogenous Pbdhfr allele was replaced with the mutant PfdhfrS108N, was generated and confirmed to have normal growth rate comparing to parental non-transgenic parasite and also confer resistance to pyrimethamine.
Conclusion: This study demonstrated the power of the transgenic P. berghei system to predict drug-resistant Pfdhfr mutations in an in vivo parasite/host setting. The system could be utilized for identification of possible novel drug-resistant mutants that could arise against new antifolate compounds and for prediction the evolution of resistance mutations.
Figures

Similar articles
-
The selectable marker human dihydrofolate reductase enables sequential genetic manipulation of the Plasmodium berghei genome.Mol Biochem Parasitol. 2000 Mar 5;106(2):199-212. doi: 10.1016/s0166-6851(99)00189-9. Mol Biochem Parasitol. 2000. PMID: 10699250
-
Transgenic Plasmodium parasites stably expressing Plasmodium vivax dihydrofolate reductase-thymidylate synthase as in vitro and in vivo models for antifolate screening.Malar J. 2011 Oct 7;10:291. doi: 10.1186/1475-2875-10-291. Malar J. 2011. PMID: 21981896 Free PMC article.
-
Identifying antimalarial compounds targeting dihydrofolate reductase-thymidylate synthase (DHFR-TS) by chemogenomic profiling.Int J Parasitol. 2016 Jul;46(8):527-35. doi: 10.1016/j.ijpara.2016.04.002. Epub 2016 May 2. Int J Parasitol. 2016. PMID: 27150044
-
Origins and spread of pfdhfr mutant alleles in Plasmodium falciparum.Acta Trop. 2010 Jun;114(3):166-70. doi: 10.1016/j.actatropica.2009.07.008. Epub 2009 Jul 14. Acta Trop. 2010. PMID: 19607799 Review.
-
The molecular basis of antifolate resistance in Plasmodium falciparum: looking beyond point mutations.Ann N Y Acad Sci. 2015 Apr;1342(1):10-8. doi: 10.1111/nyas.12662. Epub 2015 Feb 18. Ann N Y Acad Sci. 2015. PMID: 25694157 Free PMC article. Review.
Cited by
-
Evidence for pyronaridine as a highly effective partner drug for treatment of artemisinin-resistant malaria in a rodent model.Antimicrob Agents Chemother. 2014;58(1):183-95. doi: 10.1128/AAC.01466-13. Epub 2013 Oct 21. Antimicrob Agents Chemother. 2014. PMID: 24145526 Free PMC article.
-
Developmental Sensitivity in Schistosoma mansoni to Puromycin To Establish Drug Selection of Transgenic Schistosomes.Antimicrob Agents Chemother. 2018 Jul 27;62(8):e02568-17. doi: 10.1128/AAC.02568-17. Print 2018 Aug. Antimicrob Agents Chemother. 2018. PMID: 29760143 Free PMC article.
References
-
- World Health Organization. World malaria report 2009. Geneva, Switzerland: WHO Press; 2008.
-
- Sirawaraporn W, Prapunwattana P, Sirawaraporn R, Yuthavong Y, Santi DV. The dihydrofolate reductase domain of Plasmodium falciparum thymidylate synthase-dihydrofolate reductase. Gene synthesis, expression, and anti-folate-resistant mutants. J Biol Chem. 1993;268:21637–21644. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

