The δA isoform of calmodulin kinase II mediates pathological cardiac hypertrophy by interfering with the HDAC4-MEF2 signaling pathway
- PMID: 21554860
- PMCID: PMC3113443
- DOI: 10.1016/j.bbrc.2011.04.128
The δA isoform of calmodulin kinase II mediates pathological cardiac hypertrophy by interfering with the HDAC4-MEF2 signaling pathway
Abstract
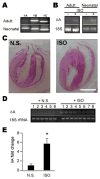
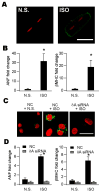
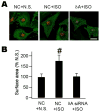
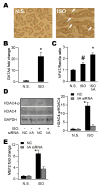
Ca(2+)/calmodulin-dependent protein kinase II (CaMKII) is a new promising target for prevention and treatment of cardiac hypertrophy and heart failure. There are three δ isoforms of CaMKII in the heart and previous studies focused primarily on δB and δC types. Here we report the δA isoform of CaMKII is also critically involved in cardiac hypertrophy. We found that δA was significantly upregulated in pathological cardiac hypertrophy in both neonatal and adult models. Upregulation of δA was accompanied by cell enlargement, sarcomere reorganization and reactivation of various hypertrophic cardiac genes including atrial natriuretic factor (ANF) and β-myocin heavy chain (β-MHC). Studies further indicated the pathological changes were largely blunted by silencing the δA gene and an underlying mechanism indicated selective interference with the HDAC4-MEF2 signaling pathway. These results provide new evidence for selective interfering cardiac hypertrophy and heart failure when CaMKII is considered as a therapeutic target.
Copyright © 2011 Elsevier Inc. All rights reserved.
Figures
References
-
- Sambrano GR, Fraser I, Han H, Ni Y, O’Connell T, Yan Z, Stull JT. Navigating the signalling network in mouse cardiac myocytes. Nature. 2002;420:712–714. - PubMed
-
- Frey N, Olson EN. Cardiac hypertrophy: the good, the bad, and the ugly. Annu Rev Physiol. 2003;65:45–79. - PubMed
-
- Bers DM. Altered cardiac myocyte Ca2+ regulation in heart failure. Physiology (Bethesda) 2006;21:380–387. - PubMed
-
- Bossuyt J, Helmstadter K, Wu X, Clements-Jewery H, Haworth RS, Avkiran M, Martin JL, Pogwizd SM, Bers DM. Ca2+/calmodulin-dependent protein kinase IIδ and protein kinase D overexpression reinforce the histone deacetylase 5 redistribution in heart failure. Circ Res. 2008;102:695–702. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

