Aristolochic acid I and ochratoxin A differentially regulate VEGF expression in porcine kidney epithelial cells--the involvement of SP-1 and HIFs transcription factors
- PMID: 21554934
- PMCID: PMC3154282
- DOI: 10.1016/j.toxlet.2011.04.022
Aristolochic acid I and ochratoxin A differentially regulate VEGF expression in porcine kidney epithelial cells--the involvement of SP-1 and HIFs transcription factors
Abstract
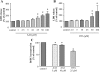
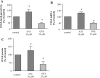
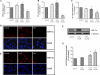
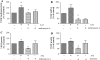
Aristolochic acid I (AAI) and ochratoxin A (OTA) cause chronic kidney diseases. Recently, the contribution of hypoxic injuries and angiogenic disturbances to nephropathies has been suggested, but underlying mechanisms have not been fully clarified yet. In porcine kidney epithelial cell line, LLC-PK1 cells, treatment with non-toxic doses of AAI increased whereas with OTA decreased production of vascular endothelial growth factor (VEGF), the angiogenic factor with well-defined functions in kidney. Moreover, the activity of transcription factors regulating VEGF expression was differentially affected by examined compounds. Activity of hypoxia inducible factors (HIFs) and SP-1 was increased by AAI but diminished by OTA. Interestingly, AP-1 activity was inhibited while NFκB was not influenced by both toxins. Mithramycin A, a SP-1 inhibitor, as well as chetomin, an inhibitor of HIFs, reversed AAI-induced up-regulation of VEGF synthesis, indicating the importance of SP-1 and HIFs in this effect. Additionally, adenoviral overexpression of HIF-2α but not HIF-1α prevented OTA-diminished VEGF production suggesting the protective effect of this isoform towards the consequences exerted by OTA. These observations provide new insight into complex impact of AAI and OTA on angiogenic gene regulation. Additionally, it adds to our understanding of hypoxia influence on nephropathies pathology.
Copyright © 2011 Elsevier Ireland Ltd. All rights reserved.
Figures
Similar articles
-
Lenticular cytoprotection. Part 1: the role of hypoxia inducible factors-1α and -2α and vascular endothelial growth factor in lens epithelial cell survival in hypoxia.Mol Vis. 2013;19:1-15. Epub 2013 Jan 2. Mol Vis. 2013. PMID: 23335846 Free PMC article.
-
Topotecan inhibits vascular endothelial growth factor production and angiogenic activity induced by hypoxia in human neuroblastoma by targeting hypoxia-inducible factor-1alpha and -2alpha.Mol Cancer Ther. 2008 Jul;7(7):1974-84. doi: 10.1158/1535-7163.MCT-07-2059. Mol Cancer Ther. 2008. PMID: 18645007
-
Ochratoxin A upregulates biomarkers associated with hypoxia and transformation in human kidney cells.Toxicol In Vitro. 2019 Jun;57:211-216. doi: 10.1016/j.tiv.2019.03.016. Epub 2019 Mar 12. Toxicol In Vitro. 2019. PMID: 30876885
-
Ochratoxin A and aristolochic acid involvement in nephropathies and associated urothelial tract tumours.Arh Hig Rada Toksikol. 2009 Dec;60(4):465-83. doi: 10.2478/10004-1254-60-2009-2000. Arh Hig Rada Toksikol. 2009. PMID: 20061248 Review.
-
Regulation of iron metabolism by hypoxia-inducible factors.Sheng Li Xue Bao. 2017 Oct 25;69(5):598-610. Sheng Li Xue Bao. 2017. PMID: 29063108 Review.
Cited by
-
Non-carcinogenic/non-nephrotoxic aristolochic acid IVa exhibited anti-inflammatory activities in mice.J Nat Med. 2023 Mar;77(2):251-261. doi: 10.1007/s11418-022-01665-8. Epub 2022 Dec 16. J Nat Med. 2023. PMID: 36525161
-
A reassessment of risk associated with dietary intake of ochratoxin A based on a lifetime exposure model.Crit Rev Toxicol. 2012 Feb;42(2):147-68. doi: 10.3109/10408444.2011.636342. Crit Rev Toxicol. 2012. PMID: 22276591 Free PMC article. Review.
-
Hypoxia, oxidative stress, and immune evasion: a trinity of the trichothecenes T-2 toxin and deoxynivalenol (DON).Arch Toxicol. 2021 Jun;95(6):1899-1915. doi: 10.1007/s00204-021-03030-2. Epub 2021 Mar 25. Arch Toxicol. 2021. PMID: 33765170 Review.
-
Blocking TGF-β Signaling Pathway Preserves Mitochondrial Proteostasis and Reduces Early Activation of PDGFRβ+ Pericytes in Aristolochic Acid Induced Acute Kidney Injury in Wistar Male Rats.PLoS One. 2016 Jul 5;11(7):e0157288. doi: 10.1371/journal.pone.0157288. eCollection 2016. PLoS One. 2016. PMID: 27379382 Free PMC article.
References
-
- Arlt V.M., Stiborova M., Schmeiser H.H. Aristolochic acid as a probable human cancer hazard in herbal remedies: a review. Mutagenesis. 2002;17:265–277. - PubMed
-
- Arlt V.M., Zuo J., Trenz K., Roufosse C.A., Lord G.M., Nortier J.L., Schmeiser H.H., Hollstein M., Phillips D.H. Gene expression changes induced by the human carcinogen aristolochic acid I in renal and hepatic tissue of mice. Int. J. Cancer. 2011;128:21–32. - PubMed
-
- Baderca F., Lighezan R., Dema A., Alexa A., Raica M. Immunohistochemical expression of VEGF in normal human renal parenchyma. Rom. J. Morphol. Embryol. 2006;47:315–322. - PubMed
-
- Boesch-Saadatmandi C., Loboda A., Jozkowicz A., Huebbe P., Blank R., Wolffram S., Dulak J., Rimbach G. Effect of ochratoxin A on redox-regulated transcription factors, antioxidant enzymes and glutathione-S-transferase in cultured kidney tubulus cells. Food Chem. Toxicol. 2008;46:2665–2671. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

