Activity of all JNK isoforms contributes to neurite growth in spiral ganglion neurons
- PMID: 21554942
- PMCID: PMC3152600
- DOI: 10.1016/j.heares.2011.04.011
Activity of all JNK isoforms contributes to neurite growth in spiral ganglion neurons
Abstract
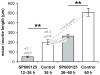
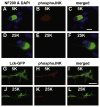
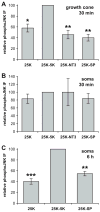
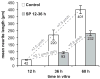
Jun N-terminal kinase (JNK) is a multifunctional protein kinase crucial for neuronal apoptosis as well as neurite growth. We have previously shown that JNK activity is correlated with spiral ganglion neuron (SGN) apoptosis following hair cell loss in rats (Alam et al., 2007) implying that JNK inhibition may have therapeutic potential to protect SGNs in deaf individuals. Here we investigated the role of JNK in neurite outgrowth from cultured neonatal rat and mouse SGNs. We show that JNK is required for initial growth of neurites and for continued extension of already established neurites. The effect of JNK inhibition on neurite growth is rapid and is also rapidly reversible after washout of the inhibitor. Using phosphoJNK immunoreactivity as an indicator, we show that JNK is activated in growth cones within 30 min after transfer to medium lacking neurotrophic stimuli (5 K medium) but activation in the nucleus and soma requires hours. By transfecting epitope-tagged JNK1, JNK2, or JNK3 isoforms into SGNs, we found that all are present in the nucleus and cytoplasm and that there is no preferential redistribution to the nucleus after transfer to 5 K medium. Cotransfection of dominant-negative (dn) JNK1 and JNK2 into SGNs reduced neurite growth, although transfection of dnJNK1 or dnJNK2 alone had no significant effect. SGNs cultured from JNK3(-/-) mice showed reduced neurite growth that was further reduced by transfection of dnJNK1 and dnJNK2. This indicates that all three JNK isoforms promote SGN neurite growth although there may be functional redundancy between JNK1 and JNK2.
Copyright © 2011 Elsevier B.V. All rights reserved.
Figures



References
-
- Alam SA, Robinson BK, Huang J, Green SH. Prosurvival and proapoptotic intracellular signaling in rat spiral ganglion neurons in vivo after the loss of hair cells. J Comp Neurol. 2007;503:832–852. - PubMed
-
- Amagasaki K, Kaneto H, Heldin CH, Lennartsson J. c-Jun N-terminal kinase is necessary for platelet-derived growth factor-mediated chemotaxis in primary fibroblasts. J Biol Chem. 2006;281:22173–9. - PubMed
-
- Benediktsson AM, Schachtele SJ, Green SH, Dailey ME. Ballistic labeling and dynamic imaging of astrocytes in organotypic hippocampal slice cultures. J Neurosci Methods. 2005;141:41–53. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

