Nox4 regulates Nrf2 and glutathione redox in cardiomyocytes in vivo
- PMID: 21554947
- PMCID: PMC3112490
- DOI: 10.1016/j.freeradbiomed.2011.04.022
Nox4 regulates Nrf2 and glutathione redox in cardiomyocytes in vivo
Abstract
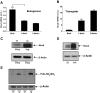
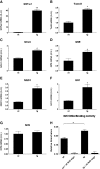
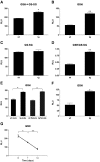
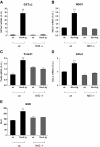
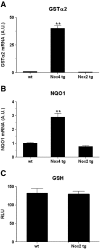
NADPH oxidase-4 (Nox4) is an important modulator of redox signaling that is inducible at the level of transcriptional expression in multiple cell types. By contrast to other Nox enzymes, Nox4 is continuously active without requiring stimulation. We reported recently that expression of Nox4 is induced in the adult heart as an adaptive stress response to pathophysiological insult. To elucidate the potential downstream target(s) regulated by Nox4, we performed a microarray screen to assess the transcriptomes of transgenic (tg) mouse hearts in which Nox4 was overexpressed. The screen revealed a significant increase in the expression of many antioxidant and detoxifying genes regulated by Nrf2 in tg compared to wild-type (wt) mouse hearts, and this finding was subsequently confirmed by Q-PCR. Expression of glutathione biosynthetic and recycling enzymes was increased in tg hearts and associated with higher levels of both GSH and the ratio of reduced:oxidised GSH, compared to wt hearts. The increases in expression of the antioxidant genes and the changes in glutathione redox effected by Nox4 were ablated in an Nrf2-null genetic background. These data therefore demonstrate that Nox4 can activate the Nrf2-regulated pathway, and suggest a potential role for Nox4 in the regulation of GSH redox in cardiomyocytes.
Copyright © 2011 Elsevier Inc. All rights reserved.
Figures

References
-
- Bedard K., Krause K.H. The NOX family of ROS-generating NADPH oxidases: physiology and pathophysiology. Physiol. Rev. 2007;87:245–313. - PubMed
-
- Lambeth J.D. NOX enzymes and the biology of reactive oxygen. Nat. Rev. Immunol. 2004;4:181–189. - PubMed
-
- Anilkumar N., Weber R., Zhang M., Brewer A., Shah A.M. Nox4 and nox2 NADPH oxidases mediate distinct cellular redox signaling responses to agonist stimulation. Arterioscler. Thromb. Vasc. Biol. 2008;28:1347–1354. - PubMed
-
- Helmcke I., Heumuller S., Tikkanen R., Schroder K., Brandes R.P. Identification of structural elements in Nox1 and Nox4 controlling localization and activity. Antioxid. Redox Signal. 2009;11:1279–1287. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

