Excitatory amplification through divergent-convergent circuits: the role of the midline thalamus in limbic seizures
- PMID: 21554957
- PMCID: PMC4297203
- DOI: 10.1016/j.nbd.2011.04.017
Excitatory amplification through divergent-convergent circuits: the role of the midline thalamus in limbic seizures
Abstract
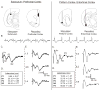
Introduction: The midline thalamic nuclei are an important component of limbic seizures. Although the anatomic connections and excitatory influences of the midline thalamus are well known, its physiological role in limbic seizures is unclear. We examined the role of the midline thalamus on two circuits that are involved in limbic seizures: (a) the subiculum-prefrontal cortex (SB-PFC), and (b) the piriform cortex-entorhinal cortex (PC-EC).
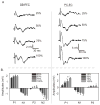
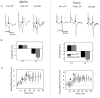
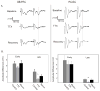
Methods: Evoked field potentials for both circuits were obtained in anesthetized rats, and the likely direct monosynaptic and polysynaptic contributions to the responses were identified. Seizures were generated in both circuits by 20 Hz stimulus trains. Once stable seizures and evoked potentials were established, the midline thalamus was inactivated through an injection of the sodium channel blocker tetrodotoxin (TTX), and the effects on the evoked responses and seizures were analyzed.
Results: Inactivation of the midline thalamus suppressed seizures in both circuits. Seizure suppression was associated with a significant reduction in the late thalamic component but no significant change in the early direct monosynaptic component. Injections that did not suppress the seizures did not alter the evoked potentials.
Conclusions: Suppression of the late thalamic component of the evoked potential at the time of seizure suppression suggests that the thalamus facilitates seizure induction by extending the duration of excitatory drive through a divergent-convergent excitatory amplification system. This work may have broader implications for understanding signaling in the limbic system.
Copyright © 2011. Published by Elsevier Inc.
Conflict of interest statement
The authors have no conflict of interest to declare.
Figures



References
-
- Avoli M, Gloor P. Interaction of cortex and thalamus in spike and wave discharges of feline generalized penicillin epilepsy. Exp Neurol. 1982;76:196–217. - PubMed
-
- Bazhenov M, Timofeev I, Steriade M, Sejnowski TJ. Cellular and network models of for intrathalamic augmenting responses during 10 Hz. stimulation. J Neurophysiol. 1998;79:2730–2748. - PubMed
-
- Berendse HW, Groenewegen HJ. Restricted cortical termination fields of the midline and intralaminar thalamic nuclei in the rat. Neuroscience. 1991;42:73–102. - PubMed
-
- Bertram EH. Functional anatomy of spontaneous seizures in a rat model of limbic epilepsy. Epilepsia. 1997;38:95–105. - PubMed
-
- Bertram EH, Zhang DX, Mangan P, Fountain N, Rempe D. Functional anatomy of limbic epilepsy: a proposal for central synchronization of a diffusely hyperexcitable network. Epilepsy Res. 1998;32:194–205. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

