GABAA receptor trafficking-mediated plasticity of inhibitory synapses
- PMID: 21555068
- PMCID: PMC3093971
- DOI: 10.1016/j.neuron.2011.03.024
GABAA receptor trafficking-mediated plasticity of inhibitory synapses
Abstract
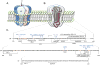
Proper developmental, neural cell-type-specific, and activity-dependent regulation of GABAergic transmission is essential for virtually all aspects of CNS function. The number of GABA(A) receptors in the postsynaptic membrane directly controls the efficacy of GABAergic synaptic transmission. Thus, regulated trafficking of GABA(A) receptors is essential for understanding brain function in both health and disease. Here we summarize recent progress in the understanding of mechanisms that allow dynamic adaptation of cell surface expression and postsynaptic accumulation and function of GABA(A) receptors. This includes activity-dependent and cell-type-specific changes in subunit gene expression, assembly of subunits into receptors, as well as exocytosis, endocytic recycling, diffusion dynamics, and degradation of GABA(A) receptors. In particular, we focus on the roles of receptor-interacting proteins, scaffold proteins, synaptic adhesion proteins, and enzymes that regulate the trafficking and function of receptors and associated proteins. In addition, we review neuropeptide signaling pathways that affect neural excitability through changes in GABA(A)R trafficking.
Copyright © 2011 Elsevier Inc. All rights reserved.
Conflict of interest statement
Figures



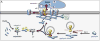
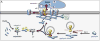
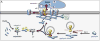

References
-
- Andang M, Lendahl U. Ion fluxes and neurotransmitters signaling in neural development. Current opinion in neurobiology. 2008;18:232–236. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

