The many faces of tau
- PMID: 21555069
- PMCID: PMC3319390
- DOI: 10.1016/j.neuron.2011.04.009
The many faces of tau
Abstract
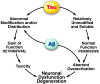
While the microtubule-binding capacity of the protein tau has been known for many years, new functions of tau in signaling and cytoskeletal organization have recently emerged. In this review, we highlight these functions and the potential roles of tau in neurodegenerative disease. We also discuss the therapeutic potential of drugs targeting various aspects of tau biology.
Copyright © 2011 Elsevier Inc. All rights reserved.
Figures


References
-
- Alonso AC, Grundke-Iqbal I, Iqbal K. Alzheimer's disease hyperphosphorylated tau sequesters normal tau into tangles of filaments and disassembles microtubules. Nat Med. 1996;2:783–787. - PubMed
-
- Alvarez A, Toro R, Cáceres A, Maccioni RB. Inhibition of tau phosphorylating protein kinase cdk5 prevents beta-amyloid-induced neuronal death. FEBS Lett. 1999;459:421–426. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

