Epithelial cell extrusion requires the sphingosine-1-phosphate receptor 2 pathway
- PMID: 21555463
- PMCID: PMC3166871
- DOI: 10.1083/jcb.201010075
Epithelial cell extrusion requires the sphingosine-1-phosphate receptor 2 pathway
Abstract
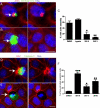
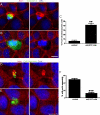
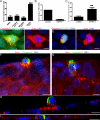
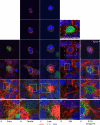
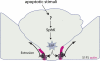
To maintain an intact barrier, epithelia eliminate dying cells by extrusion. During extrusion, a cell destined for apoptosis signals its neighboring cells to form and contract a ring of actin and myosin, which squeezes the dying cell out of the epithelium. Here, we demonstrate that the signal produced by dying cells to initiate this process is sphingosine-1-phosphate (S1P). Decreasing S1P synthesis by inhibiting sphingosine kinase activity or by blocking extracellular S1P access to its receptor prevented apoptotic cell extrusion. Extracellular S1P activates extrusion by binding the S1P(2) receptor in the cells neighboring a dying cell, as S1P(2) knockdown in these cells or its loss in a zebrafish mutant disrupted cell extrusion. Because live cells can also be extruded, we predict that this S1P pathway may also be important for driving delamination of stem cells during differentiation or invasion of cancer cells.
Figures
References
-
- Estrada R., Zeng Q., Lu H., Sarojini H., Lee J.F., Mathis S.P., Sanchez T., Wang E., Kontos C.D., Lin C.Y., et al. 2008. Up-regulating sphingosine 1-phosphate receptor-2 signaling impairs chemotactic, wound-healing, and morphogenetic responses in senescent endothelial cells. J. Biol. Chem. 283:30363–30375 10.1074/jbc.M804392200 - DOI - PMC - PubMed
-
- French K.J., Schrecengost R.S., Lee B.D., Zhuang Y., Smith S.N., Eberly J.L., Yun J.K., Smith C.D. 2003. Discovery and evaluation of inhibitors of human sphingosine kinase. Cancer Res. 63:5962–5969 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

