Lung neutrophils facilitate activation of naive antigen-specific CD4+ T cells during Mycobacterium tuberculosis infection
- PMID: 21555529
- PMCID: PMC3376160
- DOI: 10.4049/jimmunol.1100001
Lung neutrophils facilitate activation of naive antigen-specific CD4+ T cells during Mycobacterium tuberculosis infection
Abstract
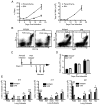
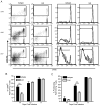
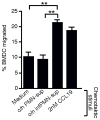
Initiation of the adaptive immune response to Mycobacterium tuberculosis occurs in the lung-draining mediastinal lymph node and requires transport of M. tuberculosis by migratory dendritic cells (DCs) to the local lymph node. The previously published observations that 1) neutrophils are a transiently prominent population of M. tuberculosis-infected cells in the lungs early in infection and 2) that the peak of infected neutrophils immediately precedes the peak of infected DCs in the lungs prompted us to characterize the role of neutrophils in the initiation of adaptive immune responses to M. tuberculosis. We found that, although depletion of neutrophils in vivo increased the frequency of M. tuberculosis-infected DCs in the lungs, it decreased trafficking of DCs to the mediastinal lymph node. This resulted in delayed activation (CD69 expression) and proliferation of naive M. tuberculosis Ag85B-specific CD4 T cells in the mediastinal lymph node. To further characterize the role of neutrophils in DC migration, we used a Transwell chemotaxis system and found that DCs that were directly infected by M. tuberculosis migrated poorly in response to CCL19, an agonist for the chemokine receptor CCR7. In contrast, DCs that had acquired M. tuberculosis through uptake of infected neutrophils exhibited unimpaired migration. These results revealed a mechanism wherein neutrophils promote adaptive immune responses to M. tuberculosis by delivering M. tuberculosis to DCs in a form that makes DCs more effective initiators of naive CD4 T cell activation. These observations provide insight into a mechanism for neutrophils to facilitate initiation of adaptive immune responses in tuberculosis.
Figures




References
-
- Meena LS, Rajni Survival mechanisms of pathogenic Mycobacterium tuberculosis H37Rv. FEBS J. 2010;277:2416–2427. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

