Pyruvate carboxylase is required for glutamine-independent growth of tumor cells
- PMID: 21555572
- PMCID: PMC3102381
- DOI: 10.1073/pnas.1016627108
Pyruvate carboxylase is required for glutamine-independent growth of tumor cells
Abstract
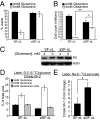
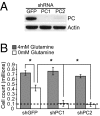
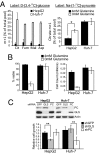
Tumor cells require a constant supply of macromolecular precursors, and interrupting this supply has been proposed as a therapeutic strategy in cancer. Precursors for lipids, nucleic acids, and proteins are generated in the tricarboxylic acid (TCA) cycle and removed from the mitochondria to participate in biosynthetic reactions. Refilling the pool of precursor molecules (anaplerosis) is therefore crucial to maintain cell growth. Many tumor cells use glutamine to feed anaplerosis. Here we studied how "glutamine-addicted" cells react to interruptions of glutamine metabolism. Silencing of glutaminase (GLS), which catalyzes the first step in glutamine-dependent anaplerosis, suppressed but did not eliminate the growth of glioblastoma cells in culture and in vivo. Profiling metabolic fluxes in GLS-suppressed cells revealed induction of a compensatory anaplerotic mechanism catalyzed by pyruvate carboxylase (PC), allowing the cells to use glucose-derived pyruvate rather than glutamine for anaplerosis. Although PC was dispensable when glutamine was available, forcing cells to adapt to low-glutamine conditions rendered them absolutely dependent on PC for growth. Furthermore, in other cell lines, measuring PC activity in nutrient-replete conditions predicted dependence on specific anaplerotic enzymes. Cells with high PC activity were resistant to GLS silencing and did not require glutamine for survival or growth, but displayed suppressed growth when PC was silenced. Thus, PC-mediated, glucose-dependent anaplerosis allows cells to achieve glutamine independence. Induction of PC during chronic suppression of glutamine metabolism may prove to be a mechanism of resistance to therapies targeting glutaminolysis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
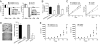

References
-
- Warburg O. On respiratory impairment in cancer cells. Science. 1956;124:269–270. - PubMed
-
- Reitzer LJ, Wice BM, Kennell D. Evidence that glutamine, not sugar, is the major energy source for cultured HeLa cells. J Biol Chem. 1979;254:2669–2676. - PubMed
-
- Tennant DA, Durán RV, Gottlieb E. Targeting metabolic transformation for cancer therapy. Nat Rev Cancer. 2010;10:267–277. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

