Continuous low-level vagus nerve stimulation reduces stellate ganglion nerve activity and paroxysmal atrial tachyarrhythmias in ambulatory canines
- PMID: 21555706
- PMCID: PMC3101282
- DOI: 10.1161/CIRCULATIONAHA.111.018028
Continuous low-level vagus nerve stimulation reduces stellate ganglion nerve activity and paroxysmal atrial tachyarrhythmias in ambulatory canines
Abstract
Background: We hypothesize that left-sided low-level vagus nerve stimulation (LL-VNS) can suppress sympathetic outflow and reduce atrial tachyarrhythmias in ambulatory dogs.
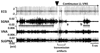
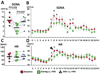
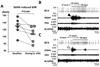
Methods and results: We implanted a neurostimulator in 12 dogs to stimulate the left cervical vagus nerve and a radiotransmitter for continuous recording of left stellate ganglion nerve activity, vagal nerve activities, and ECGs. Group 1 dogs (N=6) underwent 1 week of continuous LL-VNS. Group 2 dogs (N=6) underwent intermittent rapid atrial pacing followed by active or sham LL-VNS on alternate weeks. Integrated stellate ganglion nerve activity was significantly reduced during LL-VNS (7.8 mV/s; 95% confidence interval [CI] 6.94 to 8.66 versus 9.4 mV/s [95% CI, 8.5 to 10.3] at baseline; P=0.033) in group 1. The reduction was most apparent at 8 am, along with a significantly reduced heart rate (P=0.008). Left-sided low-level vagus nerve stimulation did not change vagal nerve activity. The density of tyrosine hydroxylase-positive nerves in the left stellate ganglion 1 week after cessation of LL-VNS were 99 684 μm(2)/mm(2) (95% CI, 28 850 to 170 517) in LL-VNS dogs and 186 561 μm(2)/mm(2) (95% CI, 154 956 to 218 166; P=0.008) in normal dogs. In group 2, the frequencies of paroxysmal atrial fibrillation and tachycardia during active LL-VNS were 1.4/d (95% CI, 0.5 to 5.1) and 8.0/d (95% CI, 5.3 to 12.0), respectively, significantly lower than during sham stimulation (9.2/d [95% CI, 5.3 to 13.1]; P=0.001 and 22.0/d [95% CI, 19.1 to 25.5], P<0.001, respectively).
Conclusions: Left-sided low-level vagus nerve stimulation suppresses stellate ganglion nerve activities and reduces the incidences of paroxysmal atrial tachyarrhythmias in ambulatory dogs. Significant neural remodeling of the left stellate ganglion is evident 1 week after cessation of continuous LL-VNS.
Figures




References
-
- Schwartz PJ, La Rovere MT, Vanoli E. Autonomic nervous system and sudden cardiac death. Experimental basis and clinical observations for post-myocardial intarction risk stratification. Circulation. 1992;85:I-77–I-91. - PubMed
-
- La Rovere MT, Pinna GD, Hohnloser SH, Marcus FI, Mortara A, Nohara R, Bigger JT, Jr, Camm AJ, Schwartz PJ. Baroreflex sensitivity and heart rate variability in the identification of patients at risk for life-threatening arrhythmias : Implications for clinical trials. Circulation. 2001;103:2072–2077. - PubMed
-
- Mortara A, La Rovere MT, Pinna GD, Parziale P, Maestri R, Capomolla S, Opasich C, Cobelli F, Tavazzi L. Depressed arterial baroreflex sensitivity and not reduced heart rate variability identifies patients with chronic heart failure and nonsustained ventricular tachycardia: The effect of high ventricular filling pressure. Am Heart J. 1997;134:879–888. - PubMed
-
- La Rovere MT, Bersano C, Gnemmi M, Specchia G, Schwartz PJ. Exercise-induced increase in baroreflex sensitivity predicts improved prognosis after myocardial infarction. Circulation. 2002;106:945–949. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

