Population pharmacokinetics of colistin methanesulfonate and formed colistin in critically ill patients from a multicenter study provide dosing suggestions for various categories of patients
- PMID: 21555763
- PMCID: PMC3122440
- DOI: 10.1128/AAC.01733-10
Population pharmacokinetics of colistin methanesulfonate and formed colistin in critically ill patients from a multicenter study provide dosing suggestions for various categories of patients
Abstract
With increasing clinical emergence of multidrug-resistant Gram-negative pathogens and the paucity of new agents to combat these infections, colistin (administered as its inactive prodrug colistin methanesulfonate [CMS]) has reemerged as a treatment option, especially for critically ill patients. There has been a dearth of pharmacokinetic (PK) data available to guide dosing in critically ill patients, including those on renal replacement therapy. In an ongoing study to develop a population PK model for CMS and colistin, 105 patients have been studied to date; these included 12 patients on hemodialysis and 4 on continuous renal replacement therapy. For patients not on renal replacement, there was a wide variance in creatinine clearance, ranging from 3 to 169 ml/min/1.73 m(2). Each patient was treated with a physician-selected CMS dosage regimen, and 8 blood samples for PK analysis were collected across a dosage interval on day 3 or 4 of therapy. A linear PK model with two compartments for CMS and one compartment for formed colistin best described the data. Covariates included creatinine clearance on the total clearance of CMS and colistin, as well as body weight on the central volume of CMS. Model-fitted parameter estimates were used to derive suggested loading and maintenance dosing regimens for various categories of patients, including those on hemodialysis and continuous renal replacement. Based on our current understanding of colistin PK and pharmacodynamic relationships, colistin may best be used as part of a highly active combination, especially for patients with moderate to good renal function and/or for organisms with MICs of ≥ 1.0 mg/liter.
Figures
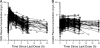


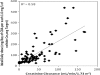
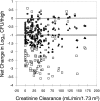
Comment in
-
Dose adjustment of polymyxins for renal insufficiency.Antimicrob Agents Chemother. 2011 Oct;55(10):4940. doi: 10.1128/AAC.05080-11. Antimicrob Agents Chemother. 2011. PMID: 21921118 Free PMC article. No abstract available.
References
-
- Antoniadou A., et al. 2007. Colistin-resistant isolates of Klebsiella pneumoniae emerging in intensive care unit patients: first report of a multiclonal cluster. J. Antimicrob. Chemother. 59:786–790 - PubMed
-
- APP Pharmaceuticals LLC 2008. Colistimethate. Package insert. APP Pharmaceuticals LLC, Schaumburg, IL
-
- Bayard D. S., Milman M. H., Schumitzky A. 1994. Design of dosage regimens: a multiple model stochastic control approach. Int. J. Biomed. Comput. 36:103–115 - PubMed
-
- Beal S. L. 2001. Ways to fit a PK model with some data below the quantification limit. J. Pharmacokinet. Pharmacodyn. 28:481–504 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

