Effects of etravirine alone and with ritonavir-boosted protease inhibitors on the pharmacokinetics of dolutegravir
- PMID: 21555764
- PMCID: PMC3122457
- DOI: 10.1128/AAC.00073-11
Effects of etravirine alone and with ritonavir-boosted protease inhibitors on the pharmacokinetics of dolutegravir
Abstract
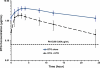
Dolutegravir (DTG) is an unboosted, once-daily integrase inhibitor currently in phase 3 trials. Two studies evaluated the effects of etravirine (ETR) alone and in combination with ritonavir (RTV)-boosted protease inhibitors (PIs) on DTG pharmacokinetics (PK) in healthy subjects. DTG 50 mg every 24 h (q24h) was administered alone for 5 days in period 1, followed by combination with ETR at 200 mg q12h for 14 days in period 2 (study 1) or with ETR/lopinavir (LPV)/RTV at 200/400/100 mg q12h or ETR/darunavir (DRV)/RTV at 200/600/100 mg q12h for 14 days in period 2 (study 2). PK samples were collected on day 5 in period 1 and day 14 in period 2. All of the treatments were well tolerated. ETR significantly decreased exposures of DTG, with geometric mean ratios of 0.294 (90% confidence intervals, 0.257 to 0.337) for the area under the curve from time zero until the end of the dosage interval (AUC(0-τ)), 0.484 (0.433 to 0.542) for the observed maximum plasma concentration (C(max)), and 0.121 (0.093 to 0.157) for the plasma concentration at the end of the dosage interval (C(τ)). ETR combined with an RTV-boosted PI affected the exposure of DTG to a lesser degree: ETR/LPV/RTV treatment had no effect on the DTG plasma AUC(0-τ) and C(max), whereas the C(τ) increased by 28%. ETR/DRV/RTV modestly decreased the plasma DTG AUC(0-τ), C(max), and C(τ) by 25, 12, and 37%, respectively. Such effects of ETR/LPV/RTV and ETR/DRV/RTV are not considered clinically relevant. The combination of DTG and ETR alone should be avoided; however, DTG may be coadministered with ETR without a dosage adjustment if LPV/RTV or DRV/RTV is concurrently administered.
Figures
References
-
- Arribas J., et al. 2010. Once-daily S/GSK1349572 as part of combination therapy in antiretroviral naive adults: rapid and potent antiviral responses in the interim 16-week analysis from SPRING-1 (ING112276), abstr. THLBB205. 18th Int. AIDS Conf.
-
- Eron J., Durant J., Poizot-Martin I. 2010. Activity of a next generation integrase inhibitor (INI), S/GSK1349572, in subjects with HIV exhibiting raltegravir resistance: initial results of VIKING study (ING112961), abstr. MOAB0105. 18th Int. AIDS Conf.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous