The p97 ATPase associates with EEA1 to regulate the size of early endosomes
- PMID: 21556036
- PMCID: PMC3271578
- DOI: 10.1038/cr.2011.80
The p97 ATPase associates with EEA1 to regulate the size of early endosomes
Abstract
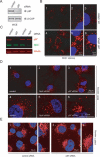
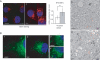
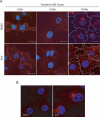
The AAA (ATPase-associated with various cellular activities) ATPase p97 acts on diverse substrate proteins to partake in various cellular processes such as membrane fusion and endoplasmic reticulum-associated degradation (ERAD). In membrane fusion, p97 is thought to function in analogy to the related ATPase NSF (N-ethylmaleimide-sensitive fusion protein), which promotes membrane fusion by disassembling a SNARE complex. In ERAD, p97 dislocates misfolded proteins from the ER membrane to facilitate their turnover by the proteasome. Here, we identify a novel function of p97 in endocytic trafficking by establishing the early endosomal autoantigen 1 (EEA1) as a new p97 substrate. We demonstrate that a fraction of p97 is localized to the early endosome membrane, where it binds EEA1 via the N-terminal C2H2 zinc finger domain. Inhibition of p97 either by siRNA or a pharmacological inhibitor results in clustering and enlargement of early endosomes, which is associated with an altered trafficking pattern for an endocytic cargo. Mechanistically, we show that p97 inhibition causes increased EEA1 self-association at the endosome membrane. We propose that p97 may regulate the size of early endosomes by governing the oligomeric state of EEA1.
Figures


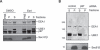

References
-
- DeLaBarre B, Brunger AT. Complete structure of p97/valosin-containing protein reveals communication between nucleotide domains. Nat Struct Biol. 2003;10:856–863. - PubMed
-
- Zhang X, Shaw A, Bates PA, et al. Structure of the AAA ATPase p97. Mol Cell. 2000;6:1473–1484. - PubMed
-
- Jentsch S, Rumpf S. Cdc48 (p97): a “molecular gearbox” in the ubiquitin pathway. Trends Biochem Sci. 2007;32:6–11. - PubMed
-
- Ye Y. Diverse functions with a common regulator: ubiquitin takes command of an AAA ATPase. J Struct Biol. 2006;156:29–40. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

