Phospho-MED1-enhanced UBE2C locus looping drives castration-resistant prostate cancer growth
- PMID: 21556051
- PMCID: PMC3116285
- DOI: 10.1038/emboj.2011.154
Phospho-MED1-enhanced UBE2C locus looping drives castration-resistant prostate cancer growth
Abstract
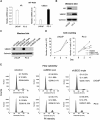
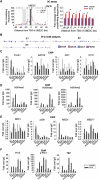
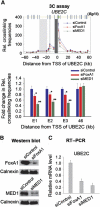
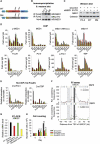
The UBE2C oncogene is overexpressed in many types of solid tumours including the lethal castration-resistant prostate cancer (CRPC). The underlying mechanisms causing UBE2C gene overexpression in CRPC are not fully understood. Here, we show that CRPC-specific enhancers drive UBE2C overexpression in both AR-negative and -positive CRPC cells. We further show that co-activator MED1 recruitment to the UBE2C enhancers is required for long-range UBE2C enhancer/promoter interactions. Importantly, we find that the molecular mechanism underlying MED1-mediated chromatin looping involves PI3K/AKT phosphorylated MED1-mediated recruitment of FoxA1, RNA polymerase II and TATA binding protein and their subsequent interactions at the UBE2C locus. MED1 phosphorylation leads to UBE2C locus looping, UBE2C gene expression and cell growth. Our results not only define a causal role of a post-translational modification (phosphorylation) of a co-activator (MED1) in forming or sustaining an active chromatin structure, but also suggest that development of specific therapies for CRPC should take account of targeting phosphorylated MED1.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures


Similar articles
-
MED1 mediates androgen receptor splice variant induced gene expression in the absence of ligand.Oncotarget. 2015 Jan 1;6(1):288-304. doi: 10.18632/oncotarget.2672. Oncotarget. 2015. PMID: 25481872 Free PMC article.
-
CCI-779 inhibits cell-cycle G2-M progression and invasion of castration-resistant prostate cancer via attenuation of UBE2C transcription and mRNA stability.Cancer Res. 2011 Jul 15;71(14):4866-76. doi: 10.1158/0008-5472.CAN-10-4576. Epub 2011 May 18. Cancer Res. 2011. PMID: 21593191 Free PMC article.
-
Transcript Levels of Androgen Receptor Variant 7 and Ubiquitin-Conjugating Enzyme 2C in Hormone Sensitive Prostate Cancer and Castration-Resistant Prostate Cancer.Prostate. 2017 Jan;77(1):60-71. doi: 10.1002/pros.23248. Epub 2016 Aug 22. Prostate. 2017. PMID: 27550197
-
Ubiquitin-conjugating enzyme E2C: a potential cancer biomarker.Int J Biochem Cell Biol. 2014 Feb;47:113-7. doi: 10.1016/j.biocel.2013.11.023. Epub 2013 Dec 17. Int J Biochem Cell Biol. 2014. PMID: 24361302 Review.
-
Estrogen receptor coactivator Mediator Subunit 1 (MED1) as a tissue-specific therapeutic target in breast cancer.J Zhejiang Univ Sci B. 2019 May;20(5):381-390. doi: 10.1631/jzus.B1900163. J Zhejiang Univ Sci B. 2019. PMID: 31090264 Free PMC article. Review.
Cited by
-
Expression of UbcH10 in pancreatic ductal adenocarcinoma and its correlation with prognosis.Tumour Biol. 2013 Jun;34(3):1473-7. doi: 10.1007/s13277-013-0671-9. Epub 2013 Jan 26. Tumour Biol. 2013. PMID: 23355337
-
Anticancer effect of icaritin on prostate cancer via regulating miR-381-3p and its target gene UBE2C.Cancer Med. 2019 Dec;8(18):7833-7845. doi: 10.1002/cam4.2630. Epub 2019 Oct 24. Cancer Med. 2019. PMID: 31646760 Free PMC article.
-
The miR 495-UBE2C-ABCG2/ERCC1 axis reverses cisplatin resistance by downregulating drug resistance genes in cisplatin-resistant non-small cell lung cancer cells.EBioMedicine. 2018 Sep;35:204-221. doi: 10.1016/j.ebiom.2018.08.001. Epub 2018 Aug 23. EBioMedicine. 2018. Retraction in: EBioMedicine. 2021 Jan;63:103168. doi: 10.1016/j.ebiom.2020.103168. PMID: 30146342 Free PMC article. Retracted.
-
Transcriptional coactivator MED1 in the interface of anti-estrogen and anti-HER2 therapeutic resistance.Cancer Drug Resist. 2022 Jun 1;5(2):498-510. doi: 10.20517/cdr.2022.33. eCollection 2022. Cancer Drug Resist. 2022. PMID: 35800368 Free PMC article. Review.
-
The lncRNA HOTAIR transcription is controlled by HNF4α-induced chromatin topology modulation.Cell Death Differ. 2019 May;26(5):890-901. doi: 10.1038/s41418-018-0170-z. Epub 2018 Aug 28. Cell Death Differ. 2019. PMID: 30154449 Free PMC article.
References
-
- Barski A, Cuddapah S, Cui K, Roh TY, Schones DE, Wang Z, Wei G, Chepelev I, Zhao K (2007) High-resolution profiling of histone methylations in the human genome. Cell 129: 823–837 - PubMed
-
- Basu S, Totty NF, Irwin MS, Sudol M, Downward J (2003) Akt phosphorylates the Yes-associated protein, YAP, to induce interaction with 14-3-3 and attenuation of p73-mediated apoptosis. Mol Cell 11: 11–23 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

