Engineering biosynthetic excitable tissues from unexcitable cells for electrophysiological and cell therapy studies
- PMID: 21556054
- PMCID: PMC3388000
- DOI: 10.1038/ncomms1302
Engineering biosynthetic excitable tissues from unexcitable cells for electrophysiological and cell therapy studies
Abstract
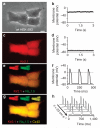
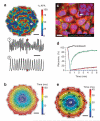
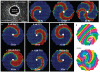
Patch-clamp recordings in single-cell expression systems have been traditionally used to study the function of ion channels. However, this experimental setting does not enable assessment of tissue-level function such as action potential (AP) conduction. Here we introduce a biosynthetic system that permits studies of both channel activity in single cells and electrical conduction in multicellular networks. We convert unexcitable somatic cells into an autonomous source of electrically excitable and conducting cells by stably expressing only three membrane channels. The specific roles that these expressed channels have on AP shape and conduction are revealed by different pharmacological and pacing protocols. Furthermore, we demonstrate that biosynthetic excitable cells and tissues can repair large conduction defects within primary 2- and 3-dimensional cardiac cell cultures. This approach enables novel studies of ion channel function in a reproducible tissue-level setting and may stimulate the development of new cell-based therapies for excitable tissue repair.
Figures

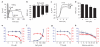

References
-
- Hille B. Ion Channels of Excitable Membranes. Sinauer; 2001.
-
- Catterall WA. The molecular basis of neuronal excitability. Science. 1984;223:653–661. - PubMed
-
- Kleber AG, Rudy Y. Basic mechanisms of cardiac impulse propagation and associated arrhythmias. Physiol. Rev. 2004;84:431–488. - PubMed
-
- Lloyd-Jones D, et al. Heart disease and stroke statistics--2010 update: a report from the American Heart Association. Circulation. 2010;121:e46–e215. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

