Biosynthesis, asymmetric synthesis, and pharmacology, including cellular targets, of the pyrrole-2-aminoimidazole marine alkaloids
- PMID: 21556392
- PMCID: PMC5596510
- DOI: 10.1039/c0np00013b
Biosynthesis, asymmetric synthesis, and pharmacology, including cellular targets, of the pyrrole-2-aminoimidazole marine alkaloids
Abstract
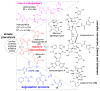
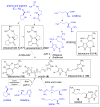
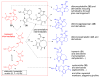
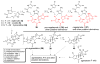
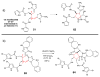
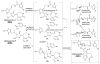
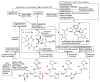
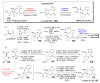
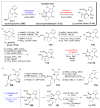
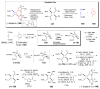
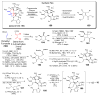
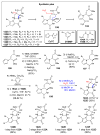
The pyrrole-2-aminoimidazole (P-2-AI) alkaloids are a growing family of marine alkaloids, now numbering well over 150 members, with high topographical and biological information content. Their intriguing structural complexity, rich and compact stereochemical content, high N to C ratio (~1 : 2), and increasingly studied biological activities are attracting a growing number of researchers from numerous disciplines world-wide. This review surveys advances in this area with a focus on the structural diversity, biosynthetic hypotheses with increasing, but still rare, verifying experimental studies, asymmetric syntheses, and biological studies, including cellular target receptor isolation studies, of this stimulating and exciting alkaloid family.
Figures













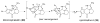











References
-
- Miller SJ, Clardy J. Nat Chem. 2009;1:261. - PubMed
-
- Andrade P, Willoughby R, Pomponi S, Kerr R. Tetrahedron Lett. 1999;40:4775.
-
- Braekman JC, Daloze D, Stoller C, van Soest RWM. Biochem Syst Ecol. 1992;20:417.
-
- Walker RP, Faulkner DJ, Van Engen D, Clardy J. J Am Chem Soc. 1981;103:6772.
-
- Keifer P, Schwartz R, Moustapha LD, Koker ES, Hughes RG, Rittschof D, Rinehart KL. J Org Chem. 1991;56:2965.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

