Anion currents in yeast K+ transporters (TRK) characterize a structural homologue of ligand-gated ion channels
- PMID: 21556692
- PMCID: PMC3151154
- DOI: 10.1007/s00424-011-0959-9
Anion currents in yeast K+ transporters (TRK) characterize a structural homologue of ligand-gated ion channels
Abstract
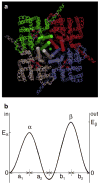
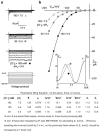
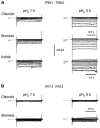
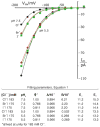
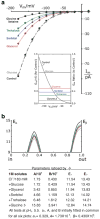
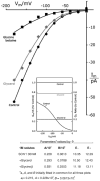
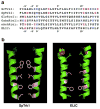
Patch clamp studies of the potassium-transport proteins TRK1,2 in Saccharomyces cerevisiae have revealed large chloride efflux currents: at clamp voltages negative to -100 mV, and intracellular chloride concentrations >10 mM (J. Membr. Biol. 198:177, 2004). Stationary-state current-voltage analysis led to an in-series two-barrier model for chloride activation: the lower barrier (α) being 10-13 kcal/mol located ~30% into the membrane from the cytoplasmic surface; and the higher one (β) being 12-16 kcal/mol located at the outer surface. Measurements carried out with lyotrophic anions and osmoprotective solutes have now demonstrated the following new properties: (1) selectivity for highly permeant anions changes with extracellular pH; at pH(o)= 5.5: I(-)≈ Br(-) >Cl(-) >SCN(-) >NO (3)(-) , and at pH(o) 7.5: I(-)≈ Br(-) > SCN(-) > NO(3)(-) >Cl(-). (2) NO(2)(-) acts like "superchoride", possibly enhancing the channel's intrinsic permeability to Cl(-). (3) SCN(-) and NO(3)(-) block chloride permeability. (4) The order of selectivity for several slightly permeant anions (at pH(o)= 5.5 only) is formate>gluconate>acetate>>phosphate(-1). (5) All anion conductances are modulated (choked) by osmoprotective solutes. (6) The data and descriptive two-barrier model evoke a hypothetical structure (Biophys. J. 77:789, 1999) consisting of an intramembrane homotetramer of fungal TRK molecules, arrayed radially around a central cluster of four single helices (TM7) from each monomer. (7) That tetrameric cluster would resemble the hydrophobic core of (pentameric) ligand-gated ion channels, and would suggest voltage-modulated hydrophobic gating to underlie anion permeation.
Figures


References
-
- Back JF, Oakenfull D, Smith MB. Increased thermal stability of proteins in the presence of sugars and polyols. Biochem. 1979;18:5191–5196. - PubMed
-
- Beckstein O, Biggin PC, Sansom MSP. A hydrophobic gating mechanism for nanopores. J Phys Chem B. 2001;105:12902–12905.
-
- Beckstein O, Sansom MSP. A hydrophobic gate in an ion channel: the closed state of nicotinic acetylcholine receptor. Phys Biol. 2006;3:147–159. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

