Hooks and comets: The story of microtubule polarity orientation in the neuron
- PMID: 21557497
- PMCID: PMC3151545
- DOI: 10.1002/dneu.20818
Hooks and comets: The story of microtubule polarity orientation in the neuron
Abstract
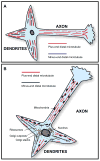
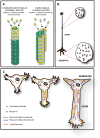
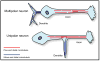
It is widely believed that signature patterns of microtubule polarity orientation within axons and dendrites underlie compositional and morphological differences that distinguish these neuronal processes from one another. Axons of vertebrate neurons display uniformly plus-end-distal microtubules, whereas their dendrites display non-uniformly oriented microtubules. Recent studies on insect neurons suggest that it is the minus-end-distal microtubules that are the critical feature of the dendritic microtubule array, whether or not they are accompanied by plus-end-distal microtubules. Discussed in this article are the history of these findings, their implications for the regulation of neuronal polarity across the animal kingdom, and potential mechanisms by which neurons establish the distinct microtubule polarity patterns that define axons and dendrites.
Copyright © 2010 Wiley Periodicals, Inc.
Figures

References
-
- Ahmad FJ, He Y, Myers KA, Hasaka TP, Francis F, Black MM, Baas PW. Effects of dynactin disruption and dynein depletion on axonal microtubules. Traffic. 2006;7:524–537. - PubMed
-
- Akhmanova A, Steinmetz MO. Tracking the ends: a dynamic protein network controls the fate of microtubule tips. Nat Rev Mol Cell Biol. 2008;9:309–322. - PubMed
-
- Alberts B. Molecular biology of the cell. Vol. 16. New York: Garland Science; 2008. pp. 965–1052.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

