The role of Rho GTPase proteins in CNS neuronal migration
- PMID: 21557504
- PMCID: PMC3188326
- DOI: 10.1002/dneu.20850
The role of Rho GTPase proteins in CNS neuronal migration
Abstract
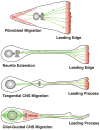
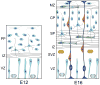
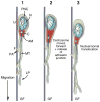
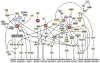
The architectonics of the mammalian brain arise from a remarkable range of directed cell migrations, which orchestrate the emergence of cortical neuronal layers and pattern brain circuitry. At different stages of cortical histogenesis, specific modes of cell motility are essential to the stepwise formation of cortical architecture. These movements range from interkinetic nuclear movements in the ventricular zone, to migrations of early-born, postmitotic polymorphic cells into the preplate, to the radial migration of precursors of cortical output neurons across the thickening cortical wall, and the vast, tangential migrations of interneurons from the basal forebrain into the emerging cortical layers. In all cases, actomyosin motors act in concert with cell adhesion receptor systems to provide the force and traction needed for forward movement. As key regulators of actin and microtubule cytoskeletons, cell polarity, and adhesion, the Rho GTPases play critical roles in CNS neuronal migration. This review will focus on the different types of migration in the developing neocortex and cerebellar cortex, and the role of the Rho GTPases, their regulators and effectors in these CNS migrations, with particular emphasis on their involvement in radial migration.
Copyright © 2010 Wiley Periodicals, Inc.
Figures

Similar articles
-
Roles of Rho small GTPases in the tangentially migrating neurons.Histol Histopathol. 2014 Jul;29(7):871-9. doi: 10.14670/HH-29.871. Epub 2014 Feb 13. Histol Histopathol. 2014. PMID: 24523202 Review.
-
Spatiotemporal Regulation of Rho GTPases in Neuronal Migration.Cells. 2019 Jun 10;8(6):568. doi: 10.3390/cells8060568. Cells. 2019. PMID: 31185627 Free PMC article. Review.
-
Molecular mechanisms of cell polarity in a range of model systems and in migrating neurons.Mol Cell Neurosci. 2020 Jul;106:103503. doi: 10.1016/j.mcn.2020.103503. Epub 2020 May 30. Mol Cell Neurosci. 2020. PMID: 32485296 Review.
-
Rap1 GTPases Are Master Regulators of Neural Cell Polarity in the Developing Neocortex.Cereb Cortex. 2017 Feb 1;27(2):1253-1269. doi: 10.1093/cercor/bhv341. Cereb Cortex. 2017. PMID: 26733533
-
RhoA and Cdc42 are required in pre-migratory progenitors of the medial ganglionic eminence ventricular zone for proper cortical interneuron migration.Development. 2013 Aug;140(15):3139-45. doi: 10.1242/dev.092585. Development. 2013. PMID: 23861058 Free PMC article.
Cited by
-
Loss of the neuron-specific F-box protein FBXO41 models an ataxia-like phenotype in mice with neuronal migration defects and degeneration in the cerebellum.J Neurosci. 2015 Jun 10;35(23):8701-17. doi: 10.1523/JNEUROSCI.2133-14.2015. J Neurosci. 2015. PMID: 26063905 Free PMC article.
-
Interkinetic nuclear migration: beyond a hallmark of neurogenesis.Cell Mol Life Sci. 2012 Aug;69(16):2727-38. doi: 10.1007/s00018-012-0952-2. Epub 2012 Mar 14. Cell Mol Life Sci. 2012. PMID: 22415322 Free PMC article. Review.
-
Ccm3, a gene associated with cerebral cavernous malformations, is required for neuronal migration.Development. 2014 Mar;141(6):1404-15. doi: 10.1242/dev.093526. Development. 2014. PMID: 24595293 Free PMC article.
-
Protein prenylation and synaptic plasticity: implications for Alzheimer's disease.Mol Neurobiol. 2014 Aug;50(1):177-85. doi: 10.1007/s12035-013-8627-z. Epub 2014 Jan 5. Mol Neurobiol. 2014. PMID: 24390573 Free PMC article. Review.
-
RhoA-ROCK Signaling as a Therapeutic Target in Traumatic Brain Injury.Cells. 2020 Jan 18;9(1):245. doi: 10.3390/cells9010245. Cells. 2020. PMID: 31963704 Free PMC article. Review.
References
-
- Adams NC, Tomoda T, Cooper M, Dietz G, Hatten ME. Mice that lack astrotactin have slowed neuronal migration. Development. 2002;129:965–972. - PubMed
-
- Amano M, Ito M, Kimura K, Fukata Y, Chihara K, Nakano T, Matsuura Y, Kaibuchi K. Phosphorylation and activation of myosin by Rho-associated kinase (Rho-kinase) J Biol Chem. 1996;271:20246–20249. - PubMed
-
- Anderson SA, Eisenstat DD, Shi L, Rubenstein JL. Interneuron migration from basal forebrain to neocortex: dependence on Dlx genes. Science. 1997;278:474–476. - PubMed
-
- Anthony TE, Klein C, Fishell G, Heintz N. Radial glia serve as neuronal progenitors in all regions of the central nervous system. Neuron. 2004;41:881–890. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

