The actin nucleating Arp2/3 complex contributes to the formation of axonal filopodia and branches through the regulation of actin patch precursors to filopodia
- PMID: 21557512
- PMCID: PMC3154400
- DOI: 10.1002/dneu.20907
The actin nucleating Arp2/3 complex contributes to the formation of axonal filopodia and branches through the regulation of actin patch precursors to filopodia
Abstract
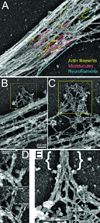
The emergence of axonal filopodia is the first step in the formation of axon collateral branches. In vitro, axonal filopodia emerge from precursor cytoskeletal structures termed actin patches. However, nothing is known about the cytoskeletal dynamics of the axon leading to the formation of filopodia in the relevant tissue environment. In this study we investigated the role of the actin nucleating Arp2/3 complex in the formation of sensory axon actin patches, filopodia, and branches. By combining in ovo chicken embryo electroporation mediated gene delivery with a novel acute ex vivo spinal cord preparation, we demonstrate that actin patches form along sensory axons and give rise to filopodia in situ. Inhibition of Arp2/3 complex function in vitro and in vivo decreases the number of axonal filopodia. In vitro, Arp2/3 complex subunits and upstream regulators localize to actin patches. Analysis of the organization of actin filaments in actin patches using platinum replica electron microscopy reveals that patches consist of networks of actin filaments, and filaments in axonal filopodia exhibit an organization consistent with the Arp2/3-based convergent elongation mechanism. Nerve growth factor (NGF) promotes formation of axonal filopodia and branches through phosphoinositide 3-kinase (PI3K). Inhibition of the Arp2/3 complex impairs NGF/PI3K-induced formation of axonal actin patches, filopodia, and the formation of collateral branches. Collectively, these data reveal that the Arp2/3 complex contributes to the formation of axon collateral branches through its involvement in the formation of actin patches leading to the emergence of axonal filopodia.
Copyright © 2011 Wiley Periodicals, Inc.
Conflict of interest statement
Conflict of Interest: none
Figures


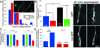
References
-
- Abosch A, Lagenaur C. Sensitivity of neurite outgrowth to microfilament disruption varies with adhesion molecule substrate. J Neurobiol. 1993;24:344–355. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

