Enantioconvergent synthesis of (+)-aphanorphine via asymmetric Pd-catalyzed alkene carboamination
- PMID: 21557571
- PMCID: PMC3107030
- DOI: 10.1021/ol2009895
Enantioconvergent synthesis of (+)-aphanorphine via asymmetric Pd-catalyzed alkene carboamination
Abstract
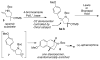
A concise asymmetric synthesis of (+)-aphanorphine has been achieved via a new enantioconvergent strategy. A racemic γ-aminoalkene derivative is transformed into a 1:1 mixture of enantiomerically enriched diastereomers using an asymmetric Pd-catalyzed carboamination. This mixture is then converted to an enantiomerically enriched protected aphanorphine derivative by a Friedel-Crafts reaction, which generates a quaternary all-carbon stereocenter. The natural product is obtained in three additional steps.
Figures
References
-
- Gulavita N, Hori A, Shimizu Y, Laszlo P, Clardy J. Tetrahedron Lett. 1988;29:4381–4384.
-
-
For recent syntheses of (−)-aphanorphine, see: Medjahdi M, González-Gómez JC, Foubelo F, Yus M. Eur Org Chem. 2011:2230–2234.Donets PA, Goeman JL, Van der Eycken J, Robeyns K, Van Meervelt L, Van der Eycken EV. Eur J Org Chem. 2009:793–796.Yang X, Cheng B, Li Z, Zhai H. Synlett. 2008:2821–2822.Yang X, Zhai H, Li Z. Org Lett. 2008;10:2457–2460.Grainger RS, Welsh EJ. Angew Chem Int Ed. 2007;46:5377–5380.Li M, Zhou P, Roth HF. Synthesis. 2007:55–60.Bower JF, Szeto P, Gallagher T. Org Biomol Chem. 2007;5:143–150.
-
-
-
For syntheses of (−)-aphanorphine prior to 2007, see: Takano S, Inomata K, Sato T, Takahashi M, Ogasawara K. J Chem Soc, Chem Commun. 1990:290–292.Bower JF, Szeto P, Gallagher T. Chem Commun. 2005:5793–5795.and references cited therein.
-
-
-
For syntheses of (+)-aphanorphine, see reference 2a and: Ma Z, Zhai H. Synlett. 2007:161–163.Ma Z, Hu H, Xiong W, Zhai H. Tetrahedron. 2007;63:7523–7531.Katoh M, Inoue H, Honda T. Heterocycles. 2007;72:497–516.Katoh M, Inoue H, Suzuki A, Honda T. Synlett. 2005:2820–2822.Meyers AI, Schmidt W, Santiago B. Heterocycles. 1995;40:525–529.Takano S, Inomata K, Sato T, Ogasawara K. J Chem Soc, Chem Commun. 1989:1591–1592.
-
-
- Fadel A, Arzel P. Tetrahedron: Asymmetry. 1995;6:893–900.
- Kita Y, Futamura J, Ohba Y, Sawama Y, Ganesh JK, Fujioka H. J Org Chem. 2003;68:5917–5924. - PubMed
- Taylor SK, Ivanovic M, Simons LJ, Davis MM. Tetrahedron: Asymmetry. 2003;14:743–747.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources