The anandamide transport inhibitor AM404 reduces the rewarding effects of nicotine and nicotine-induced dopamine elevations in the nucleus accumbens shell in rats
- PMID: 21557729
- PMCID: PMC3423245
- DOI: 10.1111/j.1476-5381.2011.01467.x
The anandamide transport inhibitor AM404 reduces the rewarding effects of nicotine and nicotine-induced dopamine elevations in the nucleus accumbens shell in rats
Abstract
Background and purpose: The fatty acid amide hydrolase inhibitor URB597 can reverse the abuse-related behavioural and neurochemical effects of nicotine in rats. Fatty acid amide hydrolase inhibitors block the degradation (and thereby magnify and prolong the actions) of the endocannabinoid anandamide (AEA), and also the non-cannabinoid fatty acid ethanolamides oleoylethanolamide (OEA) and palmitoylethanolamide (PEA). OEA and PEA are endogenous ligands for peroxisome proliferator-activated receptors alpha (PPAR-α). Since recent evidence indicates that PPAR-α can modulate nicotine reward, it is unclear whether AEA plays a role in the effects of URB597 on nicotine reward.
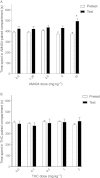
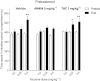
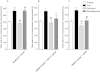
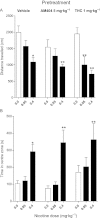
Experimental approach: A way to selectively increase endogenous levels of AEA without altering OEA or PEA levels is to inhibit AEA uptake into cells by administering the AEA transport inhibitor N-(4-hydroxyphenyl)-arachidonamide (AM404). To clarify AEA's role in nicotine reward, we investigated the effect of AM404 on conditioned place preference (CPP), reinstatement of abolished CPP, locomotor suppression and anxiolysis in an open field, and dopamine elevations in the nucleus accumbens shell induced by nicotine in Sprague-Dawley rats.
Key results: AM404 prevented the development of nicotine-induced CPP and impeded nicotine-induced reinstatement of the abolished CPP. Furthermore, AM404 reduced nicotine-induced increases in dopamine levels in the nucleus accumbens shell, the terminal area of the brain's mesolimbic reward system. AM404 did not alter the locomotor suppressive or anxiolytic effect of nicotine.
Conclusions and implications: These findings suggest that AEA transport inhibition can counteract the addictive effects of nicotine and that AEA transport may serve as a new target for development of medications for treatment of tobacco dependence.
Linked articles: This article is part of a themed section on Cannabinoids in Biology and Medicine. To view the other articles in this section visit http://dx.doi.org/10.1111/bph.2012.165.issue-8. To view Part I of Cannabinoids in Biology and Medicine visit http://dx.doi.org/10.1111/bph.2011.163.issue-7.
Published 2011. This article is a U.S. Government work and is in the public domain in the USA.
Figures

References
-
- Astarita G, Di Giacomo B, Gaetani S, Oveisi F, Compton TR, Rivara S, et al. Pharmacological characterization of hydrolysis-resistant analogs of oleoylethanolamide with potent anorexiant properties. J Pharmacol Exp Ther. 2006;318:563–570. - PubMed
-
- Beltramo M, Stella N, Calignano A, Lin SY, Makriyannis A, Piomelli D. Functional role of high-affinity anandamide transport, as revealed by selective inhibition. Science. 1997;277:1094–1097. - PubMed
-
- Bisogno T, Maccarrone M, De Petrocellis L, Jarrahian A, Finazzi-Agro A, Hillard C, et al. The uptake by cells of 2-arachidonoylglycerol, an endogenous agonist of cannabinoid receptors. Eur J Biochem. 2001;268:1982–1989. - PubMed
-
- Bortolato M, Campolongo P, Mangieri RA, Scattoni ML, Frau R, Trezza V, et al. Anxiolytic-like properties of the anandamide transport inhibitor AM404. Neuropsychopharmacology. 2006;31:2652–2659. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

