Depletion and recovery of lymphoid subsets following morphine administration
- PMID: 21557737
- PMCID: PMC3246708
- DOI: 10.1111/j.1476-5381.2011.01475.x
Depletion and recovery of lymphoid subsets following morphine administration
Abstract
Background and purpose: Opioid use and abuse has been linked to significant immunosuppression, which has been attributed, in part, to drug-induced depletion of lymphocytes. We sought to define the mechanisms by which lymphocyte populations are depleted and recover following morphine treatment in mice.
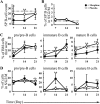
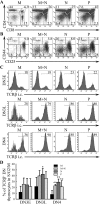
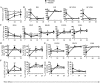
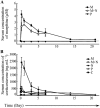
Experimental approach: Mice were implanted with morphine pellets and B- and T-cell subsets in the bone marrow, thymus, spleen and lymph nodes were analysed at various time points. We also examined the effects of morphine on T-cell development using an ex vivo assay.
Key results: The lymphocyte populations most susceptible to morphine-induced depletion were the precursor cells undergoing selection. As the lymphocytes recovered, more lymphocyte precursors proliferated than in control mice. In addition, peripheral T-cells displayed evidence that they had undergone homeostatic proliferation during the recovery phase of the experiments.
Conclusions and implications: The recovery of lymphocytes following morphine-induced depletion occurred in the presence of morphine and via increased proliferation of lymphoid precursors and homeostatic proliferation of T-cells.
© 2011 The Authors. British Journal of Pharmacology © 2011 The British Pharmacological Society.
Figures





Comment in
-
Opioids and the immune system: what is their mechanism of action?Br J Pharmacol. 2011 Dec;164(7):1826-8. doi: 10.1111/j.1476-5381.2011.01513.x. Br J Pharmacol. 2011. PMID: 21627636 Free PMC article.
References
-
- Aderjan R, Hofmann S, Schmitt G, Skopp G. Morphine and morphine glucuronides in serum of heroin consumers and in heroin-related deaths determined by HPLC with native fluorescence detection. J Anal Toxicol. 1995;19:163–168. - PubMed
-
- Arora PK, Fride E, Petitto J, Waggie K, Skolnick P. Morphine-induced immune alterations in vivo. Cell Immunol. 1990;126:343–353. - PubMed
-
- Askenasy EM, Askenasy N, Askenasy JJ. Does lymphopenia preclude restoration of immune homeostasis? The particular case of type 1 diabetes. Autoimmun Rev. 2010;9:687–690. - PubMed
-
- Ayala A, Herdon CD, Lehman DL, DeMaso CM, Ayala CA, Chaudry IH. The induction of accelerated thymic programmed cell death during polymicrobial sepsis: control by corticosteroids but not tumor necrosis factor. Shock. 1995;3:259–267. - PubMed
-
- Bach JF, Duval D, Dardenne M, Salomon JC, Tursz T, Fournier C. The effects of steroids on T-cells. Transplant Proc. 1975;7:25–30. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

