The effect of deafness duration on neurotrophin gene therapy for spiral ganglion neuron protection
- PMID: 21557994
- PMCID: PMC3152700
- DOI: 10.1016/j.heares.2011.04.010
The effect of deafness duration on neurotrophin gene therapy for spiral ganglion neuron protection
Abstract
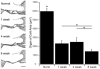
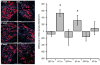
A cochlear implant can restore hearing function by electrically exciting spiral ganglion neurons (SGNs) in the deaf cochlea. However, following deafness SGNs undergo progressive degeneration ultimately leading to their death. One significant cause of SGN degeneration is the loss of neurotrophic support that is normally provided by cells within the organ of Corti (OC). The administration of exogenous neurotrophins (NTs) can protect SGNs from degeneration but the effects are short-lived once the source of NTs has been exhausted. NT gene therapy, whereby cells within the cochlea are transfected with genes enabling them to produce NTs, is one strategy for providing a cellular source of NTs that may provide long-term support for SGNs. As the SGNs normally innervate sensory cells within the OC, targeting residual OC cells for gene therapy in the deaf cochlea may provide a source of NTs for SGN protection and targeted regrowth of their peripheral fibers. However, the continual degeneration of the OC over extended periods of deafness may deplete the cellular targets for NT gene therapy and hence limit the effectiveness of this method in preventing SGN loss. This study examined the effects of deafness duration on the efficacy of NT gene therapy in preventing SGN loss in guinea pigs that were systemically deafened with aminoglycosides. Adenoviral vectors containing green fluorescent protein (GFP) with or without genes for Brain Derived Neurotrophic Factor (BDNF) and Neurotrophin-3 (NT3) were injected into the scala media (SM) compartment of cochleae that had been deafened for one, four or eight weeks prior to the viral injection. The results showed that viral transfection of cells within the SM was still possible even after severe degeneration of the OC. Supporting cells (pillar and Deiters' cells), cells within the stria vascularis, the spiral ligament, endosteal cells lining the scala compartments and interdental cells in the spiral limbus were transfected. However, the level of transfection was remarkably lower following longer durations of deafness. There was a significant increase in SGN survival in the entire basal turn for cochleae that received NT gene therapy compared to the untreated contralateral control cochleae for the one week deaf group. In the four week deaf group significant SGN survival was observed in the lower basal turn only. There was no increase in SGN survival for the eight week deaf group in any cochlear region. These findings indicated that the efficacy of NT gene therapy diminished with increasing durations of deafness leading to reduced benefits in terms of SGN protection. Clinically, there remains a window of opportunity in which NT gene therapy can provide ongoing trophic support for SGNs.
Copyright © 2011 Elsevier B.V. All rights reserved.
Figures



References
-
- Agterberg MJ, Versnel H, De Groot JC, Smoorenburg GF, Albers FW, Klis SF. Morphological changes in spiral ganglion cells after intracochlear application of brain-derived neurotrophic factor in deafened guinea pigs. Hear Res. 2008;244:25–34. - PubMed
-
- Altschuler RA, Cho Y, Ylikoski J, Pirvola U, Magal E, Miller JM. Rescue and regrowth of sensory nerves following deafferentation by neurotrophic factors. Ann N Y Acad Sci. 1999;884:305–11. - PubMed
-
- Coyne CB, Bergelson JM. CAR: a virus receptor within the tight junction. Adv Drug Deliv Rev. 2005;57:869–82. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

