Neurocognitive function in patients with recurrent glioblastoma treated with bevacizumab
- PMID: 21558074
- PMCID: PMC3107095
- DOI: 10.1093/neuonc/nor024
Neurocognitive function in patients with recurrent glioblastoma treated with bevacizumab
Abstract
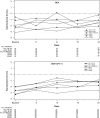
Neurocognitive decline is a frequent adverse effect of glioblastoma. Antitumor therapies that are efficacious, as measured by traditional endpoints such as objective response (OR) and progression-free survival (PFS), and have beneficial effects on neurocognitive function (NCF) are of clinical benefit to these patients. We evaluated neurocognitive changes across time in 167 patients with recurrent glioblastoma treated with bevacizumab-based therapy in BRAIN, a phase II, randomized, multicenter trial. All patients underwent MRI and neurocognitive testing at baseline and every 6 weeks thereafter. Memory, visuomotor scanning speed, and executive function were evaluated using the Hopkins Verbal Learning Test-Revised, the Trail Making Test, and the Controlled Oral Word Association test, respectively. NCF relative to baseline for patients with an OR, PFS >6 months, or disease progression was evaluated at time of OR, 24 weeks, and time of progression, respectively. For patients with an OR or PFS >6 months, median standardized test scores were examined from baseline to week 24. Most patients with an OR or PFS >6 months had poorer NCF performance compared to the general population at baseline and had improved or stable NCF at the time of response or at the 24-week assessment, respectively; most patients with progressive disease had neurocognitive decline at the time of progression. For patients with an OR or PFS >6 months, median standardized test scores were largely stable across the first 24 weeks on study. Neurocognitive testing was an objective, valid, and feasible method of monitoring NCF in patients with recurrent glioblastoma.
Figures

Comment in
-
Neurocognitive function: an emerging surrogate endpoint for neuro-oncology trials.Neuro Oncol. 2011 Jun;13(6):565. doi: 10.1093/neuonc/nor065. Neuro Oncol. 2011. PMID: 21636704 Free PMC article. No abstract available.
References
-
- Meyers CA, Hess KR, Yung WK, Levin VA. Cognitive function as a predictor of survival in patients with recurrent malignant glioma. J Clin Oncol. 2000;18:646–650. - PubMed
-
- Giovagnoli AR, Silvani A, Colombo E, Boiardi A. Facets and determinants of quality of life in patients with recurrent high grade glioma. J Neurol Neurosurg Psychiatry. 2005;76:562–568. doi:10.1136/jnnp.2004.036186. - DOI - PMC - PubMed
-
- Farace E, Shaffrey ME. Relationship of neurocognitive impairment to QOL in malignant brain tumor patients. Society for Neuro-Oncology Fifth Annual Meeting: November 9–12, 2000; Chicago, IL: 2000. Abstract 61.
-
- Meyers CA, Boake C. Neurobehavioral disorders experienced by brain tumor patients: Rehabilitation strategies. Cancer Bull. 1993;45:362–364.
-
- Tomaszewski FS, Cahn-Weiner DA, Harvey DJ, et al. Longitudinal changes in memory and executive functioning are associated with longitudinal change in instrumental activities of daily living in older adults. The Clinical Neuropsychologist. 2008;23:446–461. doi:10.1080/13854040802360558. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

