Studies of the genetics, function, and kinetic mechanism of TagE, the wall teichoic acid glycosyltransferase in Bacillus subtilis 168
- PMID: 21558268
- PMCID: PMC3129151
- DOI: 10.1074/jbc.M111.241265
Studies of the genetics, function, and kinetic mechanism of TagE, the wall teichoic acid glycosyltransferase in Bacillus subtilis 168
Abstract
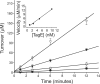
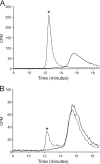
The biosynthetic enzymes involved in wall teichoic acid biogenesis in gram-positive bacteria have been the subject of renewed investigation in recent years with the benefit of modern tools of biochemistry and genetics. Nevertheless, there have been only limited investigations into the enzymes that glycosylate wall teichoic acid. Decades-old experiments in the model gram-positive bacterium, Bacillus subtilis 168, using phage-resistant mutants implicated tagE (also called gtaA and rodD) as the gene coding for the wall teichoic acid glycosyltransferase. This study and others have provided only indirect evidence to support a role for TagE in wall teichoic acid glycosylation. In this work, we showed that deletion of tagE resulted in the loss of α-glucose at the C-2 position of glycerol in the poly(glycerol phosphate) polymer backbone. We also reported the first kinetic characterization of pure, recombinant wall teichoic acid glycosyltransferase using clean synthetic substrates. We investigated the substrate specificity of TagE using a wide variety of acceptor substrates and found that the enzyme had a strong kinetic preference for the transfer of glucose from UDP-glucose to glycerol phosphate in polymeric form. Further, we showed that the enzyme recognized its polymeric (and repetitive) substrate with a sequential kinetic mechanism. This work provides direct evidence that TagE is the wall teichoic acid glycosyltransferase in B. subtilis 168 and provides a strong basis for further studies of the mechanism of wall teichoic acid glycosylation, a largely uncharted aspect of wall teichoic acid biogenesis.
Figures


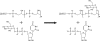
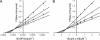
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

