Zebrafish cardiac development requires a conserved secondary heart field
- PMID: 21558385
- PMCID: PMC3091499
- DOI: 10.1242/dev.061473
Zebrafish cardiac development requires a conserved secondary heart field
Abstract
The secondary heart field is a conserved developmental domain in avian and mammalian embryos that contributes myocardium and smooth muscle to the definitive cardiac arterial pole. This field is part of the overall heart field and its myocardial component has been fate mapped from the epiblast to the heart in both mammals and birds. In this study we show that the population that gives rise to the arterial pole of the zebrafish can be traced from the epiblast, is a discrete part of the mesodermal heart field, and contributes myocardium after initial heart tube formation, giving rise to both smooth muscle and myocardium. We also show that Isl1, a transcription factor associated with undifferentiated cells in the secondary heart field in other species, is active in this field. Furthermore, Bmp signaling promotes myocardial differentiation from the arterial pole progenitor population, whereas inhibiting Smad1/5/8 phosphorylation leads to reduced myocardial differentiation with subsequent increased smooth muscle differentiation. Molecular pathways required for secondary heart field development are conserved in teleosts, as we demonstrate that the transcription factor Tbx1 and the Sonic hedgehog pathway are necessary for normal development of the zebrafish arterial pole.
Figures



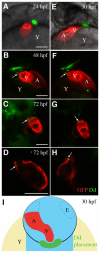
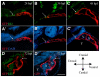
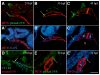

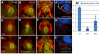
References
-
- Abu-Issa R., Kirby M. L. (2007). Heart field: from mesoderm to heart tube. Dev. Biol. 23, 45-68 - PubMed
-
- Albrecht E. W., Stegeman C. A., Heeringa P., Henning R. H., van Goor H. (2003). Protective role of endothelial nitric oxide synthase. J. Pathol. 199, 8-17 - PubMed
-
- Antonellis A., Huynh J. L., Lee-Lin S. Q., Vinton R. M., Renaud G., Loftus S. K., Elliot G., Wolfsberg T. G., Green E. D., McCallion A. S., et al. (2008). Identification of neural crest and glial enhancers at the mouse Sox10 locus through transgenesis in zebrafish. PLoS Genet. 4, e1000174 - PMC - PubMed
-
- Arguello C., de la Cruz M. V., Gomez C. S. (1975). Experimental study of the formation of the heart tube in the chick embryo. J. Embryol. Exp. Morphol. 33, 1-11 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

