A genomic predictor of response and survival following taxane-anthracycline chemotherapy for invasive breast cancer
- PMID: 21558518
- PMCID: PMC5638042
- DOI: 10.1001/jama.2011.593
A genomic predictor of response and survival following taxane-anthracycline chemotherapy for invasive breast cancer
Abstract
Context: Prediction of high probability of survival from standard cancer treatments is fundamental for individualized cancer treatment strategies.
Objective: To develop a predictor of response and survival from chemotherapy for newly diagnosed invasive breast cancer.
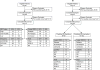
Design, setting, and patients: Prospective multicenter study conducted from June 2000 to March 2010 at the M. D. Anderson Cancer Center to develop and test genomic predictors for neoadjuvant chemotherapy. Patients were those with newly diagnosed ERBB2 (HER2 or HER2/neu)-negative breast cancer treated with chemotherapy containing sequential taxane and anthracycline-based regimens (then endocrine therapy if estrogen receptor [ER]-positive). Different predictive signatures for resistance and response to preoperative (neoadjuvant) chemotherapy (stratified according to ER status) were developed from gene expression microarrays of newly diagnosed breast cancer (310 patients). Breast cancer treatment sensitivity was then predicted using the combination of signatures for (1) sensitivity to endocrine therapy, (2) chemoresistance, and (3) chemosensitivity, with independent validation (198 patients) and comparison with other reported genomic predictors of chemotherapy response.
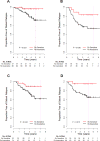
Main outcome measures: Distant relapse-free survival (DRFS) if predicted treatment sensitive and absolute risk reduction ([ARR], difference in DRFS between 2 predicted groups) at median follow-up (3 years).
Results: Patients in the independent validation cohort (99% clinical stage II-III) who were predicted to be treatment sensitive (28%) had 56% (95% CI, 31%-78%) probability of excellent pathologic response and DRFS of 92% (95% CI, 85%-100%), with an ARR of 18% (95% CI, 6%-28%). Survival was predicted in ER-positive (30% predicted sensitive; DRFS, 97% [95% CI, 91%-100%]; ARR, 11% [95% CI, 0.1%-21%]) and ER-negative (26% predicted sensitive; DRFS, 83% [95% CI, 68%-100%]; ARR, 26% [95% CI, 4%-48%]) subsets and was significant in multivariate analysis. Other genomic predictors showed paradoxically worse survival for patients predicted to be responsive to chemotherapy.
Conclusion: A genomic predictor combining ER status, predicted chemoresistance, predicted chemosensitivity, and predicted endocrine sensitivity identified patients with high probability of survival following taxane and anthracycline chemotherapy.
Conflict of interest statement
The authors have the following potential conflicts of interest to disclose:
Hatzis: Employment (Nuvera Biosciences), Equity (Nuvera Biosciences), Patents
Pusztai: Unpaid scientific advisor (Nuvera Biosciences, uncompensated), Patents
Valero: None
Booser: None
Esserman: None
Lluch: None
Vidaurre: None
Holmes: Honoraria (Novartis, Genentech, Philips) with relevance outside the submitted work
Souchon: None
Martin: None
Cotrina: None
Gomez: Honoraria (Glaxo Smith Kline, Bristol Meyers Squibb) with relevance outside the submitted work
Hubbard: None
Chacón: None
Ferrer-Lozano: None
Dyer: None
Buxton: None
Gong: None
Wu: None
Ibrahim: None
Andreopoulou: None
Ueno: None
Hunt: None
Yang: None
Nazario: None
DeMichele: None
O’Shaughnessy: None
Hortobagyi: Paid consultant (Allergan, Genentech, Novartis, SanofiAventis), research funding (Novartis), with relevance outside the submitted work
Symmans: Unpaid scientific advisor (Nuvera Biosciences), Equity (Nuvera Biosciences), Patents, Honoraria (Agendia BV).
Figures

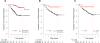

Comment in
-
Genetics: the crystal ball clears for breast cancer therapy?Nat Rev Clin Oncol. 2011 Jun 7;8(7):383. doi: 10.1038/nrclinonc.2011.91. Nat Rev Clin Oncol. 2011. PMID: 21647190 No abstract available.
-
Genomic predictor of survival after chemotherapy for breast cancer.JAMA. 2011 Aug 17;306(7):708; author reply 708-9. doi: 10.1001/jama.2011.1148. JAMA. 2011. PMID: 21846850 No abstract available.
References
-
- Sotiriou C, Pusztai L. Gene-expression signatures in breast cancer. N Engl J Med. 2009 Feb 19;360(8):790–800. - PubMed
-
- Wolmark N, Wang J, Mamounas E, Bryant J, Fisher B. Preoperative chemotherapy in patients with operable breast cancer: nine-year results from National Surgical Adjuvant Breast and Bowel Project B-18. J Natl Cancer Inst Monogr. 2001;30:96–102. - PubMed
-
- Hayes DF, Thor AD, Dressler LG, et al. HER2 and response to paclitaxel in node-positive breast cancer. N Engl J Med. 2007 Oct 11;357(15):1496–1506. - PubMed
-
- Rouzier R, Perou CM, Symmans WF, et al. Breast cancer molecular subtypes respond differently to preoperative chemotherapy. Clin Cancer Res. 2005 Aug 15;11(16):5678–5685. - PubMed
-
- Bear HD, Anderson S, Smith RE, et al. Sequential preoperative or postoperative docetaxel added to preoperative doxorubicin plus cyclophosphamide for operable breast cancer:National Surgical Adjuvant Breast and Bowel Project Protocol B-27. J Clin Oncol. 2006 May 1;24(13):2019–2027. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
Grants and funding
- CA41287/CA/NCI NIH HHS/United States
- U10 CA033601/CA/NCI NIH HHS/United States
- CA37347/CA/NCI NIH HHS/United States
- CA16450/CA/NCI NIH HHS/United States
- U10 CA041287/CA/NCI NIH HHS/United States
- CA31946/CA/NCI NIH HHS/United States
- CA33601/CA/NCI NIH HHS/United States
- CA77597/CA/NCI NIH HHS/United States
- U10 CA031946/CA/NCI NIH HHS/United States
- CA77651/CA/NCI NIH HHS/United States
- CA60138/CA/NCI NIH HHS/United States
- U10 CA077597/CA/NCI NIH HHS/United States
- R43 CA130188/CA/NCI NIH HHS/United States
- U10 CA047559/CA/NCI NIH HHS/United States
- U10 CA077651/CA/NCI NIH HHS/United States
- 1 R01 CA106290/CA/NCI NIH HHS/United States
- CA47559/CA/NCI NIH HHS/United States
- R01 CA106290/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

