Lipoxygenases mediate the effect of essential fatty acid in skin barrier formation: a proposed role in releasing omega-hydroxyceramide for construction of the corneocyte lipid envelope
- PMID: 21558561
- PMCID: PMC3129186
- DOI: 10.1074/jbc.M111.251496
Lipoxygenases mediate the effect of essential fatty acid in skin barrier formation: a proposed role in releasing omega-hydroxyceramide for construction of the corneocyte lipid envelope
Abstract
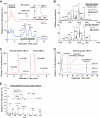
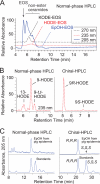
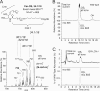
A barrier to water loss is vital to maintaining life on dry land. Formation of the mammalian skin barrier requires both the essential fatty acid linoleate and the two lipoxygenases 12R-lipoxygenase (12R-LOX) and epidermal lipoxygenase-3 (eLOX3), although their roles are poorly understood. Linoleate occurs in O-linoleoyl-ω-hydroxyceramide, which, after hydrolysis of the linoleate moiety, is covalently attached to protein via the free ω-hydroxyl of the ceramide, forming the corneocyte lipid envelope, a scaffold between lipid and protein that helps seal the barrier. Here we show using HPLC-UV, LC-MS, GC-MS, and (1)H NMR that O-linoleoyl-ω-hydroxyceramide is oxygenated in a regio- and stereospecific fashion by the consecutive actions of 12R-LOX and eLOX3 and that these products occur naturally in pig and mouse epidermis. 12R-LOX forms 9R-hydroperoxy-linoleoyl-ω-hydroxyceramide, further converted by eLOX3 to specific epoxyalcohol (9R,10R-trans-epoxy-11E-13R-hydroxy) and 9-keto-10E,12Z esters of the ceramide; an epoxy-ketone derivative (9R,10R-trans-epoxy-11E-13-keto) is the most prominent oxidized ceramide in mouse skin. These products are absent in 12R-LOX-deficient mice, which crucially display a near total absence of protein-bound ω-hydroxyceramides and of the corneocyte lipid envelope and die shortly after birth from transepidermal water loss. We conclude that oxygenation of O-linoleoyl-ω-hydroxyceramide is required to facilitate the ester hydrolysis and allow bonding of the ω-hydroxyceramide to protein, providing a coherent explanation for the roles of multiple components in epidermal barrier function. Our study uncovers a hitherto unknown biochemical pathway in which the enzymic oxygenation of ceramides is involved in building a crucial structure of the epidermal barrier.
Figures

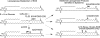



References
-
- Burr G. O., Burr M. M. (1929) J. Biol. Chem. 82, 345–367
-
- Houtsmuller U. M., van der Beek A. (1981) Prog. Lipid Res. 20, 219–224 - PubMed
-
- Akiyama M., Shimizu H. (2008) Exp. Dermatol. 17, 373–382 - PubMed
-
- Fischer J. (2009) J. Invest. Dermatol. 129, 1319–1321 - PubMed
-
- Uchida Y., Holleran W. M. (2008) J. Dermatol. Sci. 51, 77–87 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

