Stroke penumbra defined by an MRI-based oxygen challenge technique: 2. Validation based on the consequences of reperfusion
- PMID: 21559030
- PMCID: PMC3160486
- DOI: 10.1038/jcbfm.2011.67
Stroke penumbra defined by an MRI-based oxygen challenge technique: 2. Validation based on the consequences of reperfusion
Abstract
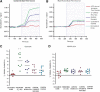
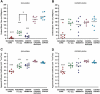
Magnetic resonance imaging (MRI) with oxygen challenge (T(2)(*) OC) uses oxygen as a metabolic biotracer to define penumbral tissue based on CMRO(2) and oxygen extraction fraction. Penumbra displays a greater T(2)(*) signal change during OC than surrounding tissue. Since timely restoration of cerebral blood flow (CBF) should salvage penumbra, T(2)(*) OC was tested by examining the consequences of reperfusion on T(2)(*) OC-defined penumbra. Transient ischemia (109 ± 20 minutes) was induced in male Sprague-Dawley rats (n=8). Penumbra was identified on T(2)(*)-weighted MRI during OC. Ischemia and ischemic injury were identified on CBF and apparent diffusion coefficient maps, respectively. Reperfusion was induced and scans repeated. T(2) for final infarct and T(2)(*) OC were run on day 7. T(2)(*) signal increase to OC was 3.4% in contralateral cortex and caudate nucleus and was unaffected by reperfusion. In OC-defined penumbra, T(2)(*) signal increased by 8.4% ± 4.1% during ischemia and returned to 3.25% ± 0.8% following reperfusion. Ischemic core T(2)(*) signal increase was 0.39% ± 0.47% during ischemia and 0.84% ± 1.8% on reperfusion. Penumbral CBF increased from 41.94 ± 13 to 116.5 ± 25 mL per 100 g per minute on reperfusion. On day 7, OC-defined penumbra gave a normal OC response and was located outside the infarct. T(2)(*) OC-defined penumbra recovered when CBF was restored, providing further validation of the utility of T(2)(*) OC for acute stroke management.
Figures



References
-
- Baron JC, Bousser MG, Comar D, Soussaline F, Castaigne R. Noninvasive tomographic study of cerebral blood flow and oxygen metabolism in vivo: potentials, limitations and clinical applications in cerebral ischemic disorders. Eur Neurol. 1981;20:273–284. - PubMed
-
- Bergstedt K, Hu BR, Wieloch T. Postischemic changes in protein synthesis in the rat brain: effects of hypothermia. Exp Brain Res. 1993;95:91–99. - PubMed
-
- Butcher KS, Parsons M, MacGregor L, Barber PA, Chalk J, Bladin C, Levi C, Kimber T, Schultz D, Fink J, Tress B, Donnan G, Davis S. Refinig the perfusion–diffusion mismatch hypothesis. Stroke. 2005;36:1153–1159. - PubMed
-
- Cocho D, Belvis R, Marti-Fabregas J, Molina-Porcel L, Diaz-Manera J, Aleu A, Pagonabarraga J, Garcia-Bargo D, Mauri A, Marti-Vilalta JL. Reasons for exclusion from thrombolytic therapy following acute ischemic stroke. Neurology. 2005;64:719–720. - PubMed
-
- Dani KA, Santosh C, Brennan D, McCabe C, Holmes WM, Condon B, Hadley DM, Macrae IM, Shaw M, Muir KW. T2* weighted magnetic resonance imaging with hyperoxia in acute ischemic stoke. Ann Neurol. 2010;68:37–47. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

