An adhesion-dependent switch between mechanisms that determine motile cell shape
- PMID: 21559321
- PMCID: PMC3086868
- DOI: 10.1371/journal.pbio.1001059
An adhesion-dependent switch between mechanisms that determine motile cell shape
Abstract
Keratocytes are fast-moving cells in which adhesion dynamics are tightly coupled to the actin polymerization motor that drives migration, resulting in highly coordinated cell movement. We have found that modifying the adhesive properties of the underlying substrate has a dramatic effect on keratocyte morphology. Cells crawling at intermediate adhesion strengths resembled stereotypical keratocytes, characterized by a broad, fan-shaped lamellipodium, clearly defined leading and trailing edges, and persistent rates of protrusion and retraction. Cells at low adhesion strength were small and round with highly variable protrusion and retraction rates, and cells at high adhesion strength were large and asymmetrical and, strikingly, exhibited traveling waves of protrusion. To elucidate the mechanisms by which adhesion strength determines cell behavior, we examined the organization of adhesions, myosin II, and the actin network in keratocytes migrating on substrates with different adhesion strengths. On the whole, our results are consistent with a quantitative physical model in which keratocyte shape and migratory behavior emerge from the self-organization of actin, adhesions, and myosin, and quantitative changes in either adhesion strength or myosin contraction can switch keratocytes among qualitatively distinct migration regimes.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
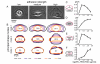



 , where
, where  is the expansion/retraction rate,
is the expansion/retraction rate,  is the rate of actin polymerization,
is the rate of actin polymerization,  is the normal component of the centripetal bulk flow
is the normal component of the centripetal bulk flow  of the viscous F-actin network, and
of the viscous F-actin network, and  is the position along the cell boundary. In a migrating cell, the actin network, myosin, and adhesions must be organized such that
is the position along the cell boundary. In a migrating cell, the actin network, myosin, and adhesions must be organized such that  is greater than
is greater than  at the front of the cell (s = 0), and
at the front of the cell (s = 0), and  is greater than
is greater than  in the cell rear (s = 100). The corners of the cell are defined by the point where
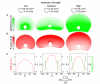
in the cell rear (s = 100). The corners of the cell are defined by the point where  (s = ±50). (B-D) Cell-substrate adhesions (green springs) oppose myosin-driven retrograde flow (blue arrows) of the actin network (red). When adhesion is strong, or contractile forces are low, the actin network is stationary with the respect to the underlying surface (
(s = ±50). (B-D) Cell-substrate adhesions (green springs) oppose myosin-driven retrograde flow (blue arrows) of the actin network (red). When adhesion is strong, or contractile forces are low, the actin network is stationary with the respect to the underlying surface ( ) and actin polymerization drives protrusion of the cell boundary (B). When adhesion is weak or contractile forces are high (C, D), the actin network moves with respect to the underlying surface (
) and actin polymerization drives protrusion of the cell boundary (B). When adhesion is weak or contractile forces are high (C, D), the actin network moves with respect to the underlying surface ( ). If the rate of polymerization is equal to the rate of retrograde flow (
). If the rate of polymerization is equal to the rate of retrograde flow ( ) then the cell boundary is stationary (C), but if actin polymerization is less than the rate of retrograde flow (
) then the cell boundary is stationary (C), but if actin polymerization is less than the rate of retrograde flow ( ) the cell boundary retracts (D).
) the cell boundary retracts (D).





References
-
- Keren K, Theriot J. A. Biophysical aspects of actin-based cell motility in fish epithelial keratocytes. Cell Motility. 2008:31–58.
-
- Gupton S. L, Waterman-Storer C. M. Spatiotemporal feedback between actomyosin and focal-adhesion systems optimizes rapid cell migration. Cell. 2006;125:1361–1374. - PubMed
-
- Palecek S. P, Loftus J. C, Ginsberg M. H, Lauffenburger D. A, Horwitz A. F. Integrin-ligand binding properties govern cell migration speed through cell-substratum adhesiveness. Nature. 1997;385:537–540. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

