Meta-analyses of adverse effects data derived from randomised controlled trials as compared to observational studies: methodological overview
- PMID: 21559325
- PMCID: PMC3086872
- DOI: 10.1371/journal.pmed.1001026
Meta-analyses of adverse effects data derived from randomised controlled trials as compared to observational studies: methodological overview
Abstract
Background: There is considerable debate as to the relative merits of using randomised controlled trial (RCT) data as opposed to observational data in systematic reviews of adverse effects. This meta-analysis of meta-analyses aimed to assess the level of agreement or disagreement in the estimates of harm derived from meta-analysis of RCTs as compared to meta-analysis of observational studies.
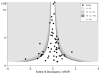
Methods and findings: Searches were carried out in ten databases in addition to reference checking, contacting experts, citation searches, and hand-searching key journals, conference proceedings, and Web sites. Studies were included where a pooled relative measure of an adverse effect (odds ratio or risk ratio) from RCTs could be directly compared, using the ratio of odds ratios, with the pooled estimate for the same adverse effect arising from observational studies. Nineteen studies, yielding 58 meta-analyses, were identified for inclusion. The pooled ratio of odds ratios of RCTs compared to observational studies was estimated to be 1.03 (95% confidence interval 0.93-1.15). There was less discrepancy with larger studies. The symmetric funnel plot suggests that there is no consistent difference between risk estimates from meta-analysis of RCT data and those from meta-analysis of observational studies. In almost all instances, the estimates of harm from meta-analyses of the different study designs had 95% confidence intervals that overlapped (54/58, 93%). In terms of statistical significance, in nearly two-thirds (37/58, 64%), the results agreed (both studies showing a significant increase or significant decrease or both showing no significant difference). In only one meta-analysis about one adverse effect was there opposing statistical significance.
Conclusions: Empirical evidence from this overview indicates that there is no difference on average in the risk estimate of adverse effects of an intervention derived from meta-analyses of RCTs and meta-analyses of observational studies. This suggests that systematic reviews of adverse effects should not be restricted to specific study types. Please see later in the article for the Editors' Summary.
Conflict of interest statement
The Academic Editor, Jan P. Vandenbroucke, has disclosed that he has worked with authors SG and YKL on another project unrelated to the study reported in this paper. He also discloses an intellectual competing interest in that he has previously published the theoretical viewpoint that estimates of harms outcomes in observational studies may be as valid as results of randomized trials, and may be more generalizable. The authors have no competing interests to declare.
Figures


References
-
- Chou R, Helfand M. Challenges in systematic reviews that assess treatment harms. Ann Intern Med. 2005;142:1090–1099. - PubMed
-
- Ioannidis JP, Mulrow CD, Goodman SN. Adverse events: the more you search, the more you find. Ann Intern Med. 2006;144:298–300. - PubMed
-
- Levine M, Walter S, Lee H, Haines T, Holbrook A, et al. User's guides to the medical literature, IV: how to use an article about harm. JAMA. 1994;271:1615–1619. - PubMed
-
- Meade MO, Cook DJ, Kernerman P, Bernard G. How to use articles about harm: the relationship between high tidal volumes, ventilating pressures, and ventilator-induced lung injury. Crit Care Med. 1997;25:1915–1922. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

