ErbB1/2 tyrosine kinase inhibitor mediates oxidative stress-induced apoptosis in inflammatory breast cancer cells
- PMID: 21559822
- PMCID: PMC3734382
- DOI: 10.1007/s10549-011-1568-1
ErbB1/2 tyrosine kinase inhibitor mediates oxidative stress-induced apoptosis in inflammatory breast cancer cells
Abstract
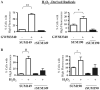
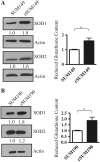
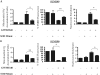
Overexpression of epidermal growth factor receptors (ErbB) is frequently seen in inflammatory breast cancer (IBC). Treatment with ErbB1/2-targeting agents (lapatinib) mediates tumor apoptosis by downregulating ErbB1/2 phosphorylation and downstream survival signaling. In this study, using carboxy-H(2)DCFDA, DHE, and MitoSOX Red to examine changes in hydrogen peroxide radicals, cytoplasmic and mitochondrial superoxide, respectively, we observed that GW583340 (a lapatinib-analog) increases reactive oxygen species (ROS) in two models of IBC (SUM149, SUM190) that are sensitive to ErbB1/2 blockade. This significant increase in ROS levels was similar to those generated by classical oxidative agents H(2)O(2) and paraquat. In contrast, minimal to basal levels of ROS were measured in a clonal population of GW583340-resistant IBC cells (rSUM149 and rSUM190). The GW583340-resistant IBC cells displayed increased SOD1, SOD2, and glutathione expression, which correlated with decreased sensitivity to the apoptotic-inducing effects of GW583340, H(2)O(2), and paraquat. The ROS increase and cell death in the GW583340-sensitive cells was reversed by simultaneous treatment with a superoxide dismutase (SOD) mimic. Additionally, overcoming the high levels of antioxidants using redox modulators induced apoptosis in the GW583340-resistant cells. Taken together, these data demonstrate a novel mechanism of lapatinib-analog-induced apoptosis and indicate that resistant cells have increased antioxidant potential, which can be overcome by treatment with SOD modulators.
Figures




References
-
- Woodward WA, Cristofanilli M. Inflammatory breast cancer. Semin Radiat Oncol. 2009;19:256–265. - PubMed
-
- Van den Eynden GG, Van der Auwera I, Van Laere S, Colpaert CG, van Dam P, Merajver S, Kleer CG, Harris AL, Van Marck EA, Dirix LY, et al. Validation of a tissue microarray to study differential protein expression in inflammatory and non-inflammatory breast cancer. Breast Cancer Res Treat. 2004;85:13–22. - PubMed
-
- Xia W, Mullin RJ, Keith BR, Liu LH, Ma H, Rusnak DW, Owens G, Alligood KJ, Spector NL. Anti-tumor activity of GW572016: a dual tyrosine kinase inhibitor blocks EGF activation of EGFR/erbB2 and downstream Erk1/2 and AKT pathways. Oncogene. 2002;21:6255–6263. - PubMed
-
- Xia W, Bisi J, Strum J, Liu L, Carrick K, Graham KM, Treece AL, Hardwicke MA, Dush M, Liao Q, et al. Regulation of survivin by ErbB2 signaling: therapeutic implications for ErbB2-overexpressing breast cancers. Cancer Res. 2006;66:1640–1647. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

