Animal models of airway diseases
- PMID: 21560049
- PMCID: PMC7121130
- DOI: 10.1007/978-94-007-1217-1_8
Animal models of airway diseases
Abstract
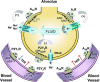
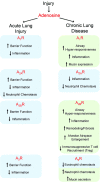
Over the past 20 years, the growing awareness that purinergic signaling events literally shape the immune and inflammatory responses to infection and allergic reactions warranted the development of animal models to assess their importance in vivo in acute lung injury and chronic airway diseases. The pioneer work conducted with the adenosine deaminase (ADA)-deficient mouse provided irrefutable evidence that excess adenosine (ADO) accumulating in the lungs of asthmatic patients, constitutes a powerful mediator of disease severity. These original studies launched the development of murine strains for the two major ectonucleotidases responsible for the generation of airway ADO from ATP release: CD39 and CD73. The dramatic acute lung injury and chronic lung complications, manifested by these knockout mice in response to allergens and endotoxin, demonstrated the critical importance of regulating the availability of ATP and ADO for their receptors. Therapeutic targets are currently evaluated using knockout mice and agonists/antagonists for each ADO receptor (A(1)R, A(2A)R, A(2B)R, and A(3)R) and the predominant ATP receptors (P2Y(2)R and P2X(7)R). This chapter provides an in-depth description of each in vivo study, and a critical view of the therapeutic potentials for the treatment of airway diseases.
Figures
Similar articles
-
Emerging Roles of Purinergic Signaling in Diabetes.Med Chem. 2018;14(5):428-438. doi: 10.2174/1573406414666180226165204. Med Chem. 2018. PMID: 29485002 Review.
-
Therapeutic applications.Subcell Biochem. 2011;55:235-76. doi: 10.1007/978-94-007-1217-1_9. Subcell Biochem. 2011. PMID: 21560050 Free PMC article. Review.
-
Purinergic signaling in wound healing and airway remodeling.Subcell Biochem. 2011;55:139-57. doi: 10.1007/978-94-007-1217-1_6. Subcell Biochem. 2011. PMID: 21560047 Review.
-
Purine Release, Metabolism, and Signaling in the Inflammatory Response.Annu Rev Immunol. 2019 Apr 26;37:325-347. doi: 10.1146/annurev-immunol-051116-052406. Epub 2019 Jan 24. Annu Rev Immunol. 2019. PMID: 30676821 Review.
-
Purinergic regulation of airway inflammation.Subcell Biochem. 2011;55:159-93. doi: 10.1007/978-94-007-1217-1_7. Subcell Biochem. 2011. PMID: 21560048 Review.
Cited by
-
CD39 and CD73 in immunity and inflammation.Trends Mol Med. 2013 Jun;19(6):355-67. doi: 10.1016/j.molmed.2013.03.005. Epub 2013 Apr 17. Trends Mol Med. 2013. PMID: 23601906 Free PMC article. Review.
-
Enhancing Extracellular Adenosine Levels Restores Barrier Function in Acute Lung Injury Through Expression of Focal Adhesion Proteins.Front Mol Biosci. 2021 Mar 10;8:636678. doi: 10.3389/fmolb.2021.636678. eCollection 2021. Front Mol Biosci. 2021. PMID: 33778007 Free PMC article.
-
Optimization of Primary Human Bronchial Epithelial 3D Cell Culture with Donor-Matched Fibroblasts and Comparison of Two Different Culture Media.Int J Mol Sci. 2023 Feb 18;24(4):4113. doi: 10.3390/ijms24044113. Int J Mol Sci. 2023. PMID: 36835529 Free PMC article.
References
-
- Balestrieri ML, Malik KU, Balestrieri C, Lee TC. Types of purinoceptors and phospholipase A2 involved in the activation of the platelet-activating factor-dependent transacetylase activity and arachidonate release by ATP in endothelial cells. Prostaglandins Other Lipid Mediat. 1998;56:363–375. - PubMed
-
- Kolosova IA, Mirzapoiazova T, Adyshev D, Usatyuk P, Romer LH, Jacobson JR, Natarajan V, Pearse DB, Garcia JGN, Verin AD. Signaling pathways involved in adenosine triphosphate-induced endothelial cell barrier enhancement. Circ Res. 2005;97:115–124. - PubMed
-
- Gunduz D, Hirche F, Hartel FV, Rodewald CW, Schafer M, Pfitzer G, Piper HM, Noll T. ATP antagonism of thrombin-induced endothelial barrier permeability. Cardiovasc Res. 2003;59:470–478. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

