Ribavirin can be mutagenic for arenaviruses
- PMID: 21561907
- PMCID: PMC3126590
- DOI: 10.1128/JVI.00614-11
Ribavirin can be mutagenic for arenaviruses
Abstract
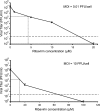
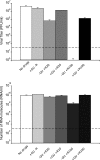
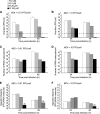
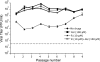
Arenaviruses include several important human pathogens, and there are very limited options of preventive or therapeutic interventions to combat these viruses. An off-label use of the purine nucleoside analogue ribavirin (1-β-d-ribofuranosyl-1-H-1,2,4-triazole-3-carboxamide) is the only antiviral treatment currently available for arenavirus infections. However, the ribavirin antiviral mechanism action against arenaviruses remains unknown. Here we document that ribavirin is mutagenic for the prototypic arenavirus lymphocytic choriomeningitis virus (LCMV) in cell culture. The mutagenic activity of ribavirin on LCMV was observed under single- and multiple-passage regimes and could not be accounted for by a decrease of the intracellular GTP pool promoted by ribavirin-mediated inhibition of inosine monophosphate dehydrogenase (IMPDH). Our findings suggest that the antiviral activity of ribavirin on arenaviruses might be exerted, at least partially, by lethal mutagenesis. Implications for antiarenavirus therapy are discussed.
Figures
References
-
- Acosta E. G., et al. 2008. Dehydroepiandrosterone, epiandrosterone and synthetic derivatives inhibit Junin virus replication in vitro. Virus Res. 135:203–212 - PubMed
-
- Agudo R., et al. 2008. Molecular characterization of a dual inhibitory and mutagenic activity of 5-fluorouridine triphosphate on viral RNA synthesis. Implications for lethal mutagenesis. J. Mol. Biol. 382:652–666 - PubMed
-
- Airaksinen A., Pariente N., Menendez-Arias L., Domingo E. 2003. Curing of foot-and-mouth disease virus from persistently infected cells by ribavirin involves enhanced mutagenesis. Virology 311:339–349 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

