A B-cell subset uniquely responsive to innate stimuli accumulates in aged mice
- PMID: 21562046
- PMCID: PMC3152496
- DOI: 10.1182/blood-2011-01-330530
A B-cell subset uniquely responsive to innate stimuli accumulates in aged mice
Abstract
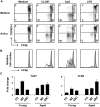
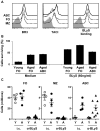
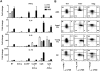
We have discovered a distinct mature B-cell subset that accumulates with age, which we have termed age-associated B cells. These cells comprise up to 30% of mature B cells by 22 months. Despite sharing some features with other mature B-cell subsets, they are refractory to BCR and CD40 stimulation. Instead, they respond to TLR9 or TLR7 stimulation and divide maximally on combined BCR and TLR ligation, leading to Ig production and preferential secretion of IL-10 and IL-4. Although similar to follicular B cells in both B-lymphocyte stimulator (BLyS) receptor expression and BLyS binding capacity, these cells do not rely on BLyS for survival. They are neither cycling nor the result of intrinsically altered B lymphopoiesis in aged BM, but instead appear to be generated from mature B cells that exhaustively expand during the individual's lifetime. Finally, they present Ag effectively and favor polarization to a TH17 profile. Together, these findings reveal that while the magnitude of the mature primary B-cell niche is maintained with age, it is increasingly occupied by cells refractory to BCR-driven activation yet responsive to innate receptor stimulation.
Figures




Comment in
-
Now you know your ABCs.Blood. 2011 Aug 4;118(5):1187-8. doi: 10.1182/blood-2011-06-355131. Blood. 2011. PMID: 21816835 No abstract available.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

