TNF-related apoptosis-inducing ligand (TRAIL) regulates inflammatory neutrophil apoptosis and enhances resolution of inflammation
- PMID: 21562052
- PMCID: PMC3644175
- DOI: 10.1189/jlb.0211062
TNF-related apoptosis-inducing ligand (TRAIL) regulates inflammatory neutrophil apoptosis and enhances resolution of inflammation
Abstract
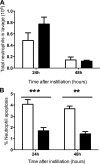
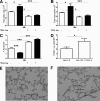
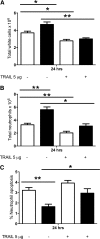
Novel therapeutics targeting neutrophilic inflammation are a major unmet clinical need in acute and chronic inflammation. The timely induction of neutrophil apoptosis is critical for inflammation resolution, and it is thought that acceleration of apoptosis may facilitate resolution at inflammatory sites. We previously demonstrated that a death receptor ligand, TRAIL, accelerates neutrophil apoptosis in vitro. We examined the role of TRAIL in neutrophil-dominant inflammation in WT and TRAIL-deficient mice. TRAIL deficiency did not alter constitutive neutrophil apoptosis, whereas exogenous TRAIL accelerated apoptosis of murine peripheral blood neutrophils. We compared TRAIL-deficient and WT mice in two independent models of neutrophilic inflammation: bacterial LPS-induced acute lung injury and zymosan-induced peritonitis. In both models, TRAIL-deficient mice had an enhanced inflammatory response with increased neutrophil numbers and reduced neutrophil apoptosis. Correction of TRAIL deficiency and supraphysiological TRAIL signaling using exogenous protein enhanced neutrophil apoptosis and reduced neutrophil numbers in both inflammatory models with no evidence of effects on other cell types. These data indicate the potential therapeutic benefit of TRAIL in neutrophilic inflammation.
Figures
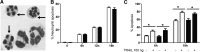




Comment in
-
Editorial: Neutrophil apoptosis: hot on the TRAIL of inflammatory resolution.J Leukoc Biol. 2011 Nov;90(5):841-3. doi: 10.1189/jlb.0511222. J Leukoc Biol. 2011. PMID: 22045921 No abstract available.
References
-
- Rowe S. J., Allen L., Ridger V. C., Hellewell P. G., Whyte M. K. (2002) Caspase-1-deficient mice have delayed neutrophil apoptosis and a prolonged inflammatory response to lipopolysaccharide-induced acute lung injury. J. Immunol. 169, 6401–6407 - PubMed
-
- Rossi A. G., Sawatzky D. A., Walker A., Ward C., Sheldrake T. A., Riley N. A., Caldicott A., Martinez-Losa M., Walker T. R., Duffin R., Gray M., Crescenzi E., Martin M. C., Brady H. J., Savill J. S., Dransfield I., Haslett C. (2006) Cyclin-dependent kinase inhibitors enhance the resolution of inflammation by promoting inflammatory cell apoptosis. Nat. Med. 12, 1056–1064 - PubMed
-
- Jones H. A., Clark R. J., Rhodes C. G., Schofield J. B., Krausz T., Haslett C. (1994) In vivo measurement of neutrophil activity in experimental lung inflammation. Am. J. Respir. Crit. Care Med. 149, 1635–1639 - PubMed
-
- Bianchi S. M., Dockrell D. H., Renshaw S. A., Sabroe I., Whyte M. K. (2006) Granulocyte apoptosis in the pathogenesis and resolution of lung disease. Clin. Sci. (Lond.) 110, 293–304 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

