Diabetes adversely affects macrophages during atherosclerotic plaque regression in mice
- PMID: 21562077
- PMCID: PMC3114401
- DOI: 10.2337/db10-0778
Diabetes adversely affects macrophages during atherosclerotic plaque regression in mice
Abstract
Objective: Patients with diabetes have increased cardiovascular risk. Atherosclerosis in these patients is often associated with increased plaque macrophages and dyslipidemia. We hypothesized that diabetic atherosclerosis involves processes that impair favorable effects of lipid reduction on plaque macrophages.
Research design and methods: Reversa mice are LDL receptor-deficient mice that develop atherosclerosis. Their elevated plasma LDL levels are lowered after conditional knockout of the gene encoding microsomal triglyceride transfer protein. We examined the morphologic and molecular changes in atherosclerotic plaques in control and streptozotocin-induced diabetic Reversa mice after LDL lowering. Bone marrow-derived macrophages were also used to study changes mediated by hyperglycemia.
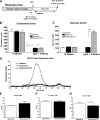
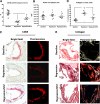
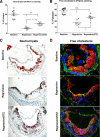
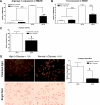
Results: Reversa mice were fed a western diet for 16 weeks to develop plaques (baseline). Four weeks after lipid normalization, control (nondiabetic) mice had reduced plasma cholesterol (-77%), plaque cholesterol (-53%), and plaque cells positive for macrophage marker CD68+ (-73%), but increased plaque collagen (+116%) compared with baseline mice. Diabetic mice had similarly reduced plasma cholesterol, but collagen content increased by only 34% compared with baseline; compared with control mice, there were lower reductions in plaque cholesterol (-30%) and CD68+ cells (-41%). Diabetic (vs. control) plaque CD68+ cells also exhibited more oxidant stress and inflammatory gene expression and less polarization toward the anti-inflammatory M2 macrophage state. Many of the findings in vivo were recapitulated by hyperglycemia in mouse bone marrow-derived macrophages.
Conclusions: Diabetes hindered plaque regression in atherosclerotic mice (based on CD68+ plaque content) and favorable changes in plaque macrophage characteristics after the reduction of elevated plasma LDL.
Figures

References
-
- Haffner SM, Lehto S, Rönnemaa T, Pyörälä K, Laakso M. Mortality from coronary heart disease in subjects with type 2 diabetes and in nondiabetic subjects with and without prior myocardial infarction. N Engl J Med 1998;339:229–234 - PubMed
-
- Karlson BW, Herlitz J, Hjalmarson A. Prognosis of acute myocardial infarction in diabetic and non-diabetic patients. Diabet Med 1993;10:449–454 - PubMed
-
- Burke AP, Kolodgie FD, Zieske A, et al. Morphologic findings of coronary atherosclerotic plaques in diabetics: a postmortem study. Arterioscler Thromb Vasc Biol 2004;24:1266–1271 - PubMed
-
- Ross R. Atherosclerosis is an inflammatory disease. Am Heart J 1999;138:S419–S420 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

