Advances in standardization of laboratory measurement procedures: implications for measuring biomarkers of folate and vitamin B-12 status in NHANES
- PMID: 21562088
- PMCID: PMC3127510
- DOI: 10.3945/ajcn.111.013359
Advances in standardization of laboratory measurement procedures: implications for measuring biomarkers of folate and vitamin B-12 status in NHANES
Abstract
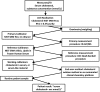
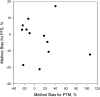
Population studies such as NHANES analyze large numbers of laboratory measurements and are often performed in different laboratories using different measurement procedures and over an extended period of time. Correct clinical and epidemiologic interpretations of the results depend on the accuracy of those measurements. Unfortunately, considerable variability has been observed among assays for folate, vitamin B-12, and related biomarkers. In the past few decades, the science of metrology has advanced considerably, with the development of improved primary reference measurement procedures and high-level reference materials, which can serve as the basis for accurate measurement. A rigorous approach has been established for making field methods traceable to the highest-level reference measurement procedures and reference materials. This article reviews some basic principles of metrology and describes their recent application to measurements of folate and vitamin B-12.
Figures

References
-
- Vesper HW, Thienpont LM. Traceability in laboratory medicine. Clin Chem 2009;55:1067–75 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

