Cytomegalovirus DNA quantification using an automated platform for nucleic acid extraction and real-time PCR assay setup
- PMID: 21562101
- PMCID: PMC3147859
- DOI: 10.1128/JCM.00721-11
Cytomegalovirus DNA quantification using an automated platform for nucleic acid extraction and real-time PCR assay setup
Abstract
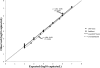
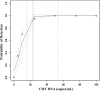
Analytical performance characteristics of the QIAsymphony RGQ system with artus cytomegalovirus (CMV) reagents were determined. Measurable range spanned 2.0 to ≥ 7.0 log(10) copies/ml. The detection limit was 23 copies/ml. Intrarun and interrun coefficients of variation were ≤ 2.1% at 3.0 and 5.0 log(10) copies/ml. In clinical specimens, RGQ values were ~0.2 log(10) copies/ml higher than those in an assay using a BioRobot M48 extraction/manual reaction setup/7500 Real-Time PCR instrument. No cross-contamination was observed.
Figures
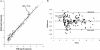
References
-
- CLSI 2010. Quantitative molecular methods for infectious diseases. Approved guideline, 2nd ed. CLSI document MM06-A2 CLSI, Wayne, PA
-
- Finney D. J. 1971. Probit analysis, 3rd ed. Cambridge University Press, New York, NY
-
- Finney D. J. 1978. Statistical method in biological assay, 3rd ed. Charles Griffin & Co., London, United Kingdom
-
- Kotton C. N., et al. 2010. International consensus guidelines on the management of cytomegalovirus in solid organ transplantation. Transplantation 89:779–795 - PubMed
-
- Ljungman P., Hakki M., Boeckh M. 2010. Cytomegalovirus in hematopoietic stem cell transplant recipients. Infect. Dis. Clin. North Am. 24:319–337 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

