Randomized, placebo-controlled clinical trial of an aerosolized β₂-agonist for treatment of acute lung injury
- PMID: 21562125
- PMCID: PMC3175548
- DOI: 10.1164/rccm.201012-2090OC
Randomized, placebo-controlled clinical trial of an aerosolized β₂-agonist for treatment of acute lung injury
Abstract
Rationale: β₂-Adrenergic receptor agonists accelerate resolution of pulmonary edema in experimental and clinical studies.
Objectives: This clinical trial was designed to test the hypothesis that an aerosolized β₂-agonist, albuterol, would improve clinical outcomes in patients with acute lung injury (ALI).
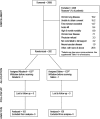
Methods: We conducted a multicenter, randomized, placebo-controlled clinical trial in which 282 patients with ALI receiving mechanical ventilation were randomized to receive aerosolized albuterol (5 mg) or saline placebo every 4 hours for up to 10 days. The primary outcome variable for the trial was ventilator-free days.
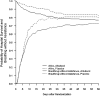
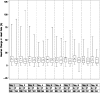
Measurements and main results: Ventilator-free days were not significantly different between the albuterol and placebo groups (means of 14.4 and 16.6 d, respectively; 95% confidence interval for the difference, -4.7 to 0.3 d; P = 0.087). Rates of death before hospital discharge were not significantly different between the albuterol and placebo groups (23.0 and 17.7%, respectively; 95%confidence interval for the difference,-4.0 to 14.7%;P = 0.30). In the subset of patients with shock before randomization, the number of ventilator-free days was lower with albuterol, although mortality was not different. Overall, heart rates were significantly higher in the albuterol group by approximately 4 beats/minute in the first 2 days after randomization, but rates of new atrial fibrillation (10% in both groups) and other cardiac dysrhythmias were not significantly different.
Conclusions: These results suggest that aerosolized albuterol does not improve clinical outcomes in patients with ALI. Routine use of β₂-agonist therapy in mechanically ventilated patients with ALI cannot be recommended. Clinical trial registered with www.clinicaltrials.gov (NCT 00434993).
Trial registration: ClinicalTrials.gov NCT00434993.
Figures
Comment in
-
Pro: β-agonists in acute lung injury--the end of the story?Am J Respir Crit Care Med. 2011 Sep 1;184(5):503-4. doi: 10.1164/rccm.201106-1115ED. Am J Respir Crit Care Med. 2011. PMID: 21885634 No abstract available.
-
Inflammation-induced desensitization of β-receptors in acute lung injury.Am J Respir Crit Care Med. 2012 Apr 15;185(8):894; author reply 894-5. doi: 10.1164/ajrccm.185.8.894. Am J Respir Crit Care Med. 2012. PMID: 22505755 No abstract available.
-
First do no harm: surrogate endpoints and the lesson of β-agonists in acute lung injury.Crit Care. 2012 Jun 26;16(3):314. doi: 10.1186/CC11392. Crit Care. 2012. PMID: 22731873 Free PMC article.
References
-
- Crandall ED, Matthay MA. Alveolar epithelial transport: basic science to clinical medicine. Am J Respir Crit Care Med 2001;163:1021–1029 - PubMed
-
- Matthay MA, Folkesson HG, Clerici C. Lung epithelial fluid transport and the resolution of pulmonary edema. Physiol Rev 2002;82:569–600 - PubMed
-
- Mutlu GM, Sznajder JI. Mechanisms of pulmonary edema clearance. Am J Physiol Lung Cell Mol Physiol 2005;289:L685–L695 - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

