The magnitudes of hyperpolarization-activated and low-voltage-activated potassium currents co-vary in neurons of the ventral cochlear nucleus
- PMID: 21562186
- PMCID: PMC3154804
- DOI: 10.1152/jn.00015.2010
The magnitudes of hyperpolarization-activated and low-voltage-activated potassium currents co-vary in neurons of the ventral cochlear nucleus
Abstract
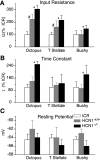
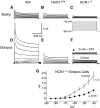
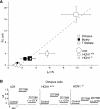
In the ventral cochlear nucleus (VCN), neurons have hyperpolarization-activated conductances, which in some cells are enormous, that contribute to the ability of neurons to convey acoustic information in the timing of their firing by decreasing the input resistance and speeding-up voltage changes. Comparisons of the electrophysiological properties of neurons in the VCN of mutant mice that lack the hyperpolarization-activated cyclic nucleotide-gated channel α subunit 1 (HCN1(-/-)) (Nolan et al. 2003) with wild-type controls (HCN1(+/+)) and with outbred ICR mice reveal that octopus, T stellate, and bushy cells maintain their electrophysiological distinctions in all strains. Hyperpolarization-activated (I(h)) currents were smaller and slower, input resistances were higher, and membrane time constants were longer in HCN1(-/-) than in HCN1(+/+) in octopus, bushy, and T stellate cells. There were significant differences in the average magnitudes of I(h), input resistances, and time constants between HCN1(+/+) and ICR mice, but the resting potentials did not differ between strains. I(h) is opposed by a low-voltage-activated potassium (I(KL)) current in bushy and octopus cells, whose magnitudes varied widely between neuronal types and between strains. The magnitudes of I(h) and I(KL) were correlated across neuronal types and across mouse strains. Furthermore, these currents balanced one another at the resting potential in individual cells. The magnitude of I(h) and I(KL) is linked in bushy and octopus cells and varies not only between HCN1(-/-) and HCN1(+/+) but also between "wild-type" strains of mice, raising the question to what extent the wild-type strains reflect normal mice.
Figures




References
-
- Adams JC. Projections from octopus cells of the posteroventral cochlear nucleus to the ventral nucleus of the lateral lemniscus in cat and human. Auditory Neurosci 3: 335–350, 1997
-
- Altomare C, Terragni B, Brioschi C, Milanesi R, Pagliuca C, Viscomi C, Moroni A, Baruscotti M, Altomare C, Terrangni B, Brioshi C, Milanesi R, Pegliuca C, Viscomi C, Moroni A, Baruscotti M, Baruscotti M, DiFrancesco D. Heteromeric HCN1-HCN4 channels: a comparison with native pacemaker channels from the rabbit sinoatrial node. J Physiol 549: 347–359, 2003 - PMC - PubMed
-
- Bal R, Baydas G, Naziroglu M. Electrophysiological properties of ventral cochlear nucleus neurons of the dog. Hear Res 256: 93–103, 2009 - PubMed
-
- Bal R, Oertel D. Hyperpolarization-activated, mixed-cation current (Ih) in octopus cells of the mammalian cochlear nucleus. J Neurophysiol 84: 806–817, 2000 - PubMed
-
- Bal R, Oertel D. Potassium currents in octopus cells of the mammalian cochlear nuclei. J Neurophysiol 86: 2299–2311, 2001 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

