Comparison of IVIg and PLEX in patients with myasthenia gravis
- PMID: 21562253
- PMCID: PMC3109880
- DOI: 10.1212/WNL.0b013e31821e5505
Comparison of IVIg and PLEX in patients with myasthenia gravis
Abstract
Objective: Both IV immunoglobulin (IVIg) and plasma exchange (PLEX) are immunomodulatory treatments used to treat patients with myasthenia gravis (MG), but the choice of which treatment to administer to patients is limited due to lack of evidence from adequately powered, masked, randomized, standardized trials.
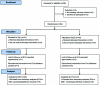
Methods: We randomized 84 patients with moderate to severe MG defined as a Quantitative Myasthenia Gravis Score for disease severity (QMGS) of >10.5 and worsening weakness to IVIg (Gamunex®, Talecris Biotherapeutics) 1 g/kg/day for 2 consecutive days or PLEX (Caridian Spectra) 1.0 plasma volume exchanges for 5 exchanges. The patients were evaluated at day 14 after treatment for the primary efficacy parameter of change in QMGS and secondary clinical and electrophysiologic parameters and were followed for a total of 60 days.
Results: Both IVIg and PLEX reduced the QMGS, and IVIg was comparable to PLEX in efficacy. The dropout rate was the same for both treatment arms and both treatments were well-tolerated. The presence of acetylcholine receptor antibodies and greater baseline disease severity predicted a better response to therapy. The postintervention status revealed that the same proportion of patients improved with treatment: 69% on IVIg and 65% on PLEX. The duration of improvement was similar with both treatments.
Conclusions: IVIg has comparable efficacy to PLEX in the treatment of patients with moderate to severe MG. Both treatments are well-tolerated, and the duration of effect is comparable. Either treatment may be offered to patients depending on availability of resources.
Classification of evidence: This study provides Class I evidence that IVIg and PLEX have comparable efficacy and are equally tolerated in adult patients with moderate to severe MG within 2 weeks of treatment.
Copyright © 2011 by AAN Enterprises, Inc.
Figures

References
-
- Vincent A, McConville J, Farrugia ME, et al. Antibodies in myasthenia gravis and related disorders. Ann NY Acad Sci 2003;998:324–335 - PubMed
-
- Vincent A, Leite MI. Neuromuscular junction autoimmune disease: muscle specific kinase antibodies and treatments for myasthenia gravis. Curr Opin Neurol 2005;18:519–525 - PubMed
-
- Vincent A, Palace J, Hilton-Jones D. Myasthenia gravis. Lancet 2001;357:2122–2128 - PubMed
-
- Hohlfeld R, Wekerle H. The immunopathogenesis of myasthenia gravis. In: Engel AG. ed. Myasthenia Gravis and Myasthenic Disorders. New York: Oxford University Press; 1999:87–104
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
