Impaired GABA and glycine transmission triggers cardinal features of rapid eye movement sleep behavior disorder in mice
- PMID: 21562273
- PMCID: PMC6703223
- DOI: 10.1523/JNEUROSCI.0347-11.2011
Impaired GABA and glycine transmission triggers cardinal features of rapid eye movement sleep behavior disorder in mice
Erratum in
- J Neurosci. 2011 Jun 22;31(25):9440
Abstract
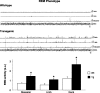
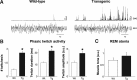
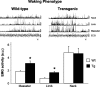
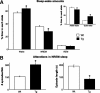
Rapid eye movement (REM) sleep behavior disorder (RBD) is a neurological disease characterized by loss of normal REM motor inhibition and subsequent dream enactment. RBD is clinically relevant because it predicts neurodegenerative disease onset (e.g., Parkinson's disease) and is clinically problematic because it disrupts sleep and results in patient injuries and hospitalization. Even though the cause of RBD is unknown, multiple lines of evidence indicate that abnormal inhibitory transmission underlies the disorder. Here, we show that transgenic mice with deficient glycine and GABA transmission have a behavioral, motor, and sleep phenotype that recapitulates the cardinal features of RBD. Specifically, we show that mice with impaired glycine and GABA(A) receptor function exhibit REM motor behaviors, non-REM muscle twitches, sleep disruption, and EEG slowing--the defining disease features. Importantly, the RBD phenotype is rescued by drugs (e.g., clonazepam and melatonin) that are routinely used to treat human disease symptoms. Our findings are the first to identify a potential mechanism for RBD--we show that deficits in glycine- and GABA(A)-mediated inhibition trigger the full spectrum of RBD symptoms. We propose that these mice are a useful resource for investigating in vivo disease mechanisms and developing potential therapeutics for RBD.
Figures





References
-
- Albin RL, Koeppe RA, Chervin RD, Consens FB, Wernette K, Frey KA, Aldrich MS. Decreased striatal dopaminergic innervation in REM sleep behavior disorder. Neurology. 2000;55:1410–1412. - PubMed
-
- Boeve BF, Silber MH, Ferman TJ. Melatonin for treatment of REM sleep behavior disorder in neurologic disorders: results in 14 patients. Sleep Med. 2003;4:281–284. - PubMed
-
- Boeve BF, Silber MH, Saper CB, Ferman TJ, Dickson DW, Parisi JE, Benarroch EE, Ahlskog JE, Smith GE, Caselli RC, Tippman-Peikert M, Olson EJ, Lin SC, Young T, Wszolek Z, Schenck CH, Mahowald MW, Castillo PR, Del Tredici K, Braak H. Pathophysiology of REM sleep behaviour disorder and relevance to neurodegenerative disease. Brain. 2007;130:2770–2788. - PubMed
-
- Braak H, Del Tredici K, Rüb U, de Vos RAI, Jansen Steur EN, Braak E. Staging of brain pathology related to sporadic Parkinson's disease. Neurobiol Aging. 2003;24:197–211. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
