Antenatal inflammation reduces expression of caveolin-1 and influences multiple signaling pathways in preterm fetal lungs
- PMID: 21562314
- PMCID: PMC3361364
- DOI: 10.1165/rcmb.2010-0519OC
Antenatal inflammation reduces expression of caveolin-1 and influences multiple signaling pathways in preterm fetal lungs
Abstract
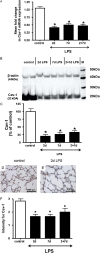
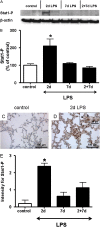
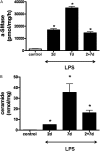
Bronchopulmonary dysplasia (BPD), associated with chorioamnionitis, results from the simultaneous effects of disrupted lung development, lung injury, and repair superimposed on the developing lung. Caveolins (Cavs) are implicated as major modulators of lung injury and remodeling by multiple signaling pathways, although Cavs have been minimally studied in the injured developing lung. We hypothesized that chorioamnionitis-associated antenatal lung inflammation would decrease the expression of Cav-1 in preterm fetal lungs. We tested whether changes occurred in the transcription factors Smad2/3, Smad1/5, Stat3, and Stat1, and we also studied the activation of acid-sphingomyelinase (a-SMase) with the generation of ceramide, along with changes in the expression of heme oxygenase-1 (HO-1) as indicators of possible Cav-1-mediated effects. Fetal sheep were exposed to 10 mg of intra-amniotic endotoxin or saline for 2, 7, or 2 + 7 days before preterm delivery at 124 days of gestation. The expression of Cav-1 and HO-1 and the phosphorylation of Smad and Stat were evaluated by real-time PCR, Western blotting, and/or immunohistochemistry. The activity of a-SMase and the concentrations of ceramide were measured. Intra-amniotic endotoxin decreased Cav-1 mRNA and protein expression in the lungs, with a maximum reduction of Cav-1 mRNA to 50% ± 7% of the control value (P < 0.05), and of Cav-1 protein expression to 20% ± 5% of the control value (P < 0.05). Decreased concentrations of Cav-1 were associated with the elevated phosphorylation of Smad2/3, Stat3, and Stat1, but not of Smad1/5. The expression of HO-1, a-SMase activity, and ceramide increased. Antenatal inflammation decreased the expression of Cav-1 in the preterm fetal lung. The decreased expression of Cav-1 was associated with the activation of the Smad2/3, Stat, and a-SMase/ceramide pathways, and with the increased expression of HO-1. The decreased concentrations of Cav-1 and changes in other signaling pathways may contribute to BPD.
Figures




Similar articles
-
Antenatal inflammation induced TGF-beta1 but suppressed CTGF in preterm lungs.Am J Physiol Lung Cell Mol Physiol. 2007 Jan;292(1):L223-31. doi: 10.1152/ajplung.00159.2006. Epub 2006 Aug 25. Am J Physiol Lung Cell Mol Physiol. 2007. PMID: 16936247
-
Antenatal glucocorticoids counteract LPS changes in TGF-β pathway and caveolin-1 in ovine fetal lung.Am J Physiol Lung Cell Mol Physiol. 2013 Mar 15;304(6):L438-44. doi: 10.1152/ajplung.00251.2012. Epub 2013 Jan 18. Am J Physiol Lung Cell Mol Physiol. 2013. PMID: 23333802 Free PMC article.
-
[Effect of intra-amniotic endotoxin priming plus hyperoxic exposure on the expression of vascular endothelial growth factor and its receptors in lungs of preterm newborn rats].Zhonghua Er Ke Za Zhi. 2007 Jul;45(7):533-8. Zhonghua Er Ke Za Zhi. 2007. PMID: 17953812 Chinese.
-
Inflammation-induced preterm lung maturation: lessons from animal experimentation.Paediatr Respir Rev. 2017 Jun;23:72-77. doi: 10.1016/j.prrv.2016.10.004. Epub 2016 Oct 20. Paediatr Respir Rev. 2017. PMID: 27856214 Review.
-
Antenatal associations with lung maturation and infection.J Perinatol. 2005 May;25 Suppl 2:S31-5. doi: 10.1038/sj.jp.7211317. J Perinatol. 2005. PMID: 15861169 Review.
Cited by
-
Caffeine modulates glucocorticoid-induced expression of CTGF in lung epithelial cells and fibroblasts.Respir Res. 2017 Mar 23;18(1):51. doi: 10.1186/s12931-017-0535-8. Respir Res. 2017. PMID: 28330503 Free PMC article.
-
The Future of Bronchopulmonary Dysplasia: Emerging Pathophysiological Concepts and Potential New Avenues of Treatment.Front Med (Lausanne). 2017 May 22;4:61. doi: 10.3389/fmed.2017.00061. eCollection 2017. Front Med (Lausanne). 2017. PMID: 28589122 Free PMC article. Review.
-
Complex roles of TGF-β signaling pathways in lung development and bronchopulmonary dysplasia.Am J Physiol Lung Cell Mol Physiol. 2023 Mar 1;324(3):L285-L296. doi: 10.1152/ajplung.00106.2021. Epub 2023 Jan 10. Am J Physiol Lung Cell Mol Physiol. 2023. PMID: 36625900 Free PMC article. Review.
-
Ceramides in tracheal aspirates of preterm infants: Marker for bronchopulmonary dysplasia.PLoS One. 2018 Jan 18;13(1):e0185969. doi: 10.1371/journal.pone.0185969. eCollection 2018. PLoS One. 2018. PMID: 29346372 Free PMC article.
-
Nebulization of Hypoxic hUCMSC-EVs Attenuates Airway Epithelial Barrier Defects in Chronic Asthma Mice by Transferring CAV-1.Int J Nanomedicine. 2024 Oct 29;19:10941-10959. doi: 10.2147/IJN.S476151. eCollection 2024. Int J Nanomedicine. 2024. PMID: 39493276 Free PMC article.
References
-
- Thomas W, Speer CP. Chorioamnionitis: important risk factor or innocent bystander for neonatal outcome? Neonatology 2010;99:177–187 - PubMed
-
- Lahra MM, Jeffery HE. A fetal response to chorioamnionitis is associated with early survival after preterm birth. Am J Obstet Gynecol 2004;190:147–151 - PubMed
-
- Yoon BH, Jun JK, Romero R, Park KH, Gomez R, Choi JH, Kim IO. Amniotic fluid inflammatory cytokines (interleukin-6, interleukin-1beta, and tumor necrosis factor–alpha), neonatal brain white matter lesions, and cerebral palsy. Am J Obstet Gynecol 1997;177:19–26 - PubMed
-
- Gantert M, Been JV, Gavilanes AW, Garnier Y, Zimmermann LJ, Kramer BW. Chorioamnionitis: a multiorgan disease of the fetus? J Perinatol 2010;30:S21–S30 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

